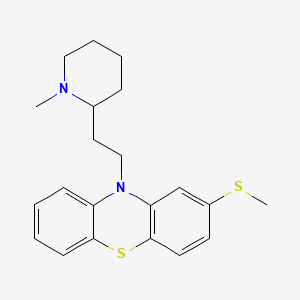
Thioridazine
Overview
Description
Thioridazine is a phenothiazine-derived antipsychotic initially approved for schizophrenia. Beyond its neuroleptic effects, it exhibits broad antimicrobial activity against pathogens like Salmonella enterica and Mycobacterium tuberculosis . Its chiral structure allows stereoisomeric differentiation: the levorotatory (–)-enantiomer demonstrates reduced neurotropism and enhanced antimicrobial efficacy compared to the racemic mixture . This compound is unique in its tissue concentration properties, enabling localized therapeutic effects . Additionally, it modulates RhoA/YAP signaling to reduce endothelial inflammation, suggesting utility in atherosclerosis . However, cardiotoxicity linked to its (+) enantiomer and metabolites led to its market withdrawal in many regions .
Preparation Methods
Thioridazine can be synthesized through various methods. One common synthetic route involves the reaction of 2-methylmercapto-10-(2-(N-methyl-2-piperidyl)ethyl)phenothiazine with appropriate reagents . The synthesis of optically pure enantiomers of this compound has been achieved using an auxiliary-based strategy, which allows for high optical purity and good overall yield . Industrial production methods often involve large-scale synthesis using similar reaction conditions but optimized for efficiency and cost-effectiveness .
Chemical Reactions Analysis
Thioridazine undergoes several types of chemical reactions, including oxidation, reduction, and substitution . Common reagents used in these reactions include oxidizing agents like potassium permanganate and reducing agents such as lithium aluminum hydride . Major products formed from these reactions include various sulfoxides and sulfone derivatives . For example, this compound can be oxidized to form mesoridazine, a metabolite with similar pharmacological properties .
Scientific Research Applications
Thioridazine is an antipsychotic drug that has been identified as having potential applications beyond its original psychiatric use. Research has explored its efficacy in treating various conditions, including cancer, tuberculosis, and inflammatory diseases .
Scientific Research Applications
Cancer Therapy:
- This compound has demonstrated anti-cancer properties in several studies. It can reduce the viability of glioblastoma multiforme (GBM) cells and GBM stem cells, induce autophagy, and affect the expression of related proteins .
- In vitro experiments have shown that this compound reduces the cell viability of gastric cancer cells, inhibits colony formation, and induces apoptosis through the mitochondrial pathway .
- In vivo studies indicate that this compound pretreatment can inhibit the growth of tumors derived from NCI-N87 gastric cancer cells in mice .
- This compound has shown more effectiveness than fluphenazine against GBM stem-like cells .
- This compound may be an effective drug for cancer therapy by targeting cancer stem cells (CSCs), which play a vital role in drug resistance, cancer relapse, and metastasis .
Tuberculosis Treatment:
- This compound has shown promise in treating both susceptible and multidrug-resistant tuberculosis (TB) .
- This compound treatment resulted in a significant reduction of colony-forming units of both susceptible and multidrug-resistant tuberculosis bacilli in the lungs of mice .
- When this compound was added to a regimen containing rifampicin, isoniazid, and pyrazinamide for susceptible tuberculosis, a significant synergistic effect was achieved .
Anti-inflammatory Agent:
- This compound has been identified as a potential anti-inflammatory agent .
- It blocks IκBα protein degradation and subsequent NF-κB activation, which inhibits inflammation .
- In LPS-injected mice, this compound demonstrated anti-inflammatory efficacy .
Data Tables and Case Studies
While the provided documents do not contain comprehensive data tables and well-documented case studies, they do offer specific experimental findings that can be summarized.
Gastric Cancer Study (in vivo):
- NCI-N87 cells were pretreated with either DMSO or 5 μM this compound for 24 hours before being injected into nude mice .
- Pretreatment with 5 μM this compound inhibited the growth of NCI-N87 cell-derived tumors .
- Tumors derived from this compound-pretreated cells were smaller and weighed less than those from DMSO-pretreated cells .
Tuberculosis Study (in vivo):
- This compound was tested as a monotherapy in a Balb/c mouse model for both susceptible and multidrug-resistant tuberculosis .
- Treatment with this compound resulted in a significant reduction in colony-forming units of both susceptible and multidrug-resistant tuberculosis bacilli in the lung .
- The dose of 70 mg/kg of this compound significantly reduced CFUs at 30 and 60 days after initiation of treatment in mice infected with an MDR-TB strain .
Safety and Adverse Effects
- Over 50% of individuals on regular this compound experienced adverse events during or following drug withdrawal .
- The most common substituted drug during withdrawal was chlorpromazine .
- This compound alone was associated with sudden unexplained death, with drug-induced arrhythmia as the likely mechanism .
- Adverse events during or after withdrawal included re-emergence of psychosis or mood disturbance, escalation of behavioral disturbance, and tardive dyskinesia .
Mechanism of Action
Thioridazine exerts its effects by blocking postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain . It also blocks alpha-adrenergic receptors, depresses the release of hypothalamic and hypophyseal hormones, and affects the reticular activating system, which influences basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis . These actions contribute to its antipsychotic and anxiolytic effects .
Comparison with Similar Compounds
Comparison with Structural Analogs
Mesoridazine
- Structural Similarity: Mesoridazine is a thioridazine analog with identical phenothiazine cores but differing side chains.
- Target Profiles : Both are multi-target ligands (MTLs) acting on dopamine, serotonin, and histamine receptors. However, in a screen of 227 targets, this compound was active against 15 unique targets (e.g., specific GPCRs), while mesoridazine acted on two distinct targets (unidentified kinases) .
- Clinical Use : Both are antipsychotics, but mesoridazine lacks this compound’s antimicrobial and anticancer applications.
Comparison with Other Phenothiazines
Chlorpromazine and Trifluoperazine
- Antipsychotic Efficacy : All three are dopamine antagonists, but this compound has higher affinity for serotonin receptors .
- Cardiotoxicity: this compound causes pronounced QT prolongation and T-wave abnormalities in 100% of patients at high doses, whereas chlorpromazine and trifluoperazine induce similar changes in 50% and 17% of patients, respectively .
Promethazine and Methdilazine
- Primary Use : Both are antihistamines; this compound is primarily an antipsychotic.
- Antitubercular Activity: this compound’s efficacy against M. tuberculosis is attributed to its neuroleptic use, which may drive resistance via prolonged exposure.
Antimicrobial Activity Compared to Non-Phenothiazines
- Mechanism : this compound disrupts bacterial membranes and inhibits efflux pumps (e.g., adeB in A. baumannii), enhancing antibiotic efficacy .
- Efficacy :
Cardiotoxicity Profile
- Enantiomer-Specific Effects : The (+) enantiomer of this compound inhibits hERG potassium channels, causing fatal arrhythmias. The (–) enantiomer retains antimicrobial activity without cardiotoxicity .
- Metabolites : this compound-5-sulfoxide, a major metabolite, prolongs Q-T intervals at 12 µM in rat hearts, mimicking clinical cardiotoxicity .
- Comparison: this compound vs. Haloperidol: Haloperidol (a butyrophenone) lacks hERG inhibition, making it safer for cardiac patients . this compound vs. Quetiapine: Quetiapine, an atypical antipsychotic, has negligible ECG effects compared to this compound’s high-risk profile .
Derivatives and Modified Compounds
- T5 Derivative :
Metabolic and Analytical Considerations
- Metabolites : this compound-5-sulfoxide exists as diastereoisomers, detectable via HPLC. Light exposure during analysis causes degradation artifacts, complicating pharmacokinetic studies .
- Comparison with Clozapine : Clozapine is light-stable, unlike this compound, simplifying its analytical protocols .
Biological Activity
Thioridazine is an antipsychotic medication that has gained attention for its diverse biological activities, particularly in the fields of oncology and pharmacology. Originally developed for the treatment of schizophrenia, recent studies have revealed its potential in inducing apoptosis in various cancer cell lines and enhancing the efficacy of chemotherapy agents.
1. Anticancer Properties
this compound exhibits significant anticancer activity through several mechanisms:
- Induction of Apoptosis : Research indicates that this compound can induce apoptosis in various cancer types, including neuroblastoma, glioma, leukemia, and breast cancer. It achieves this by downregulating anti-apoptotic proteins such as Mcl-1 and c-FLIP, which are critical for cell survival .
- Cell Cycle Regulation : this compound has been shown to affect cell cycle regulators. It downregulates cyclin D1 and CDK4 while upregulating p21 and p16, leading to G0/G1 phase arrest in ovarian cancer cells .
- Synergistic Effects with Chemotherapy : In studies combining this compound with carboplatin, a chemotherapeutic agent, it was found that this combination enhances sensitivity to treatment by promoting apoptosis through proteasomal degradation of Mcl-1 and c-FLIP. This approach also involves the upregulation of proteasome subunit alpha 5 (PSMA5) via Nrf2 activation .
Case Studies
Several case studies highlight this compound's effectiveness in cancer treatment:
- Breast Carcinoma and Glioma : A study demonstrated that the combination of this compound with carboplatin significantly increased apoptosis in MDA-MB231 (breast carcinoma) and U87MG (glioma) cells compared to treatment with carboplatin alone .
- Gastric Cancer : this compound has shown potent anti-gastric cancer effects both in vitro and in vivo. It was found to significantly inhibit cell proliferation and induce cell death through apoptosis mechanisms .
Safety Profile
Despite its promising biological activities, this compound is associated with several side effects, particularly at high doses. These include:
- Cardiac Arrhythmias : this compound has been linked to serious cardiac side effects such as dysrhythmia and sudden death, especially when used in combination with other medications .
- Drug Resistance Overcoming : this compound has been noted to overcome drug resistance mechanisms by inhibiting P-glycoprotein, which is often responsible for reduced efficacy of chemotherapeutic agents .
Summary Table of Biological Activities
| Biological Activity | Mechanism | Cancer Types Affected |
|---|---|---|
| Induction of Apoptosis | Downregulation of Mcl-1 and c-FLIP | Breast cancer, glioma, leukemia |
| Cell Cycle Arrest | Downregulation of cyclin D1; upregulation of p21 | Ovarian cancer |
| Enhanced Chemotherapy Efficacy | Synergistic effect with carboplatin | Breast carcinoma, glioma |
| Overcoming Drug Resistance | Inhibition of P-glycoprotein | Various cancers |
Q & A
Basic Research Questions
Q. What are the primary molecular targets of thioridazine, and how do they influence its antipsychotic and anticancer activities?
this compound primarily antagonizes dopamine D2 and D4 receptors, contributing to its antipsychotic effects by modulating dopaminergic signaling in the central nervous system . Additionally, it inhibits the PI3K-Akt-mTOR pathway, which is critical for its anti-angiogenic and pro-apoptotic effects in cancer cells. Researchers should validate these targets using receptor-binding assays (e.g., radioligand displacement) and pathway inhibition studies (e.g., immunoblotting for phosphorylated Akt/mTOR) .
Q. What pharmacokinetic properties of this compound are critical for designing in vivo studies?
this compound has a hepatic half-life of 21–25 hours, with extensive metabolism into active metabolites like N-desmethylthis compound and sulfoxides. Its hydrophilicity results in high plasma concentrations (hundreds of ng/mL), and it accumulates in melanin-rich tissues (e.g., eyes). Researchers should monitor hepatic function and metabolite levels using HPLC or LC-MS, particularly in chronic dosing studies .
Q. What in vitro assays are commonly used to assess this compound’s dopaminergic antagonism?
Dopamine receptor binding assays (using radiolabeled ligands like [³H]-spiperone) and functional cAMP modulation tests are standard. For example, D2 receptor antagonism can be quantified via inhibition of dopamine-induced cAMP reduction in transfected cell lines .
Advanced Research Questions
Q. How does this compound induce apoptosis in cancer cells, and what key signaling pathways are involved?
this compound upregulates pro-apoptotic PDCD4 and downregulates BCL2, CCND1, and c-MYC, inducing mitochondrial-mediated apoptosis. It also inhibits PI3K-Akt-mTOR signaling, reducing phosphorylation of downstream targets like p70S6K and 4E-BP1. Methodologies include flow cytometry (annexin V/PI staining), qPCR for gene expression, and immunoblotting for caspase-3/9 cleavage .
Q. How can researchers reconcile conflicting data on this compound’s efficacy in dementia-related psychosis across clinical trials?
Meta-analyses (e.g., ) show no superiority over placebo in elderly dementia patients, possibly due to heterogeneous study designs, unblinded allocation, or inconsistent outcome measures (e.g., behavioral vs. cognitive scales). Future trials should prioritize concealed allocation, standardized dementia assessments (e.g., ADAS-Cog), and stratification by dementia subtype .
Q. What methodological approaches are recommended for studying this compound’s anti-angiogenic effects in tumor models?
Use in vivo xenograft models to quantify CD31⁺ blood vessels via immunohistochemistry. Complement with immunoblotting for VEGF, HIF-1α, and VEGFR-2 phosphorylation. In vitro, endothelial tube formation assays and matrigel plug assays can validate anti-angiogenic activity .
Q. How does this compound’s inhibition of the PI3K-Akt-mTOR pathway contribute to its dual role in antipsychotic and anticancer effects?
In schizophrenia, PI3K-Akt-mTOR modulation may mitigate synaptic plasticity deficits. In cancer, pathway inhibition suppresses proliferation and angiogenesis. Researchers should employ phospho-specific antibodies to track pathway activity in dual-purpose studies, comparing neuronal and cancer cell lines .
Q. What experimental strategies can mitigate this compound’s cardiotoxicity while preserving therapeutic efficacy?
Computational modeling of hERG channel interactions and enantiomer-specific testing (e.g., isolated rabbit papillary muscle assays) can identify less cardiotoxic derivatives. Concurrent ECG monitoring in preclinical studies is advised to detect QTc prolongation .
Q. How can transcriptomic and survival data from platforms like DRUGSURV inform this compound’s repositioning in oncology?
DRUGSURV links this compound’s off-target effects (e.g., EGFR inhibition) to survival-associated genes in cancers like leukemia and myeloma. Researchers should validate these targets using CRISPR/Cas9 knockout models and correlate findings with patient survival data from TCGA or GEO datasets .
Q. Methodological Guidance
Q. What are the recommended quality control parameters for preparing this compound solutions in experimental settings?
Use HPLC-certified reference standards (purity >98%) and prepare stock solutions in DMSO at 10 mM. Validate stability via UV-Vis spectrophotometry and store aliquots at -80°C to prevent oxidation. Include COA (Certificate of Analysis) and SDS documentation for reproducibility .
Properties
IUPAC Name |
10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfanylphenothiazine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KLBQZWRITKRQQV-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCCCC1CCN2C3=CC=CC=C3SC4=C2C=C(C=C4)SC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H26N2S2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6023656 | |
| Record name | Thioridazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023656 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
370.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Thioridazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014817 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
BP: 230 °C at 0.02 mm Hg | |
| Record name | Thioridazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00679 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thioridazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3189 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Soluble in alcohol (1 in 6), chloroform (1 in 0.81), ether (1 in 3); freely soluble in dehydrated alcohol. Insoluble in water, Slightly soluble in acetone, In water, log units of molar solubility, mole/L = -5.82 (0.560 mg/L), 8.55e-04 g/L | |
| Record name | Thioridazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00679 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thioridazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3189 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thioridazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014817 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Crystals from acetone, Colorless crystals | |
CAS No. |
50-52-2 | |
| Record name | Thioridazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-52-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Thioridazine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050522 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Thioridazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00679 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thioridazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023656 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Thioridazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.041 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | THIORIDAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/N3D6TG58NI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Thioridazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3189 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thioridazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014817 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
72-74 °C, MP: 158-160 °C. UV max (water): 262, 310 nm (epsilon 41842, 3215); (95% ethanol): 264, 310 nm (epsilon 41598, 3256); (0.1N HCl): 264, 305 nm (epsilon 42371, 5495): (0.1N NaOH): 263 nm (epsilon 18392). Soluble in water (1 in 9); freely soluble in ethanol (1 in 10), methanol, chloroform (1 in 5). Insoluble in ether /Thioridazine hydrochloride/, 73 °C | |
| Record name | Thioridazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00679 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Thioridazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3189 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thioridazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014817 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















