Stavudine
Description
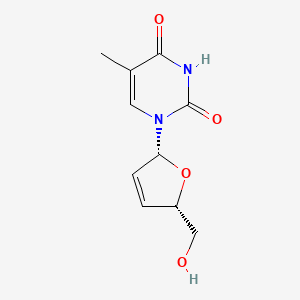
Stavudine is a nucleoside analogue obtained by formal dehydration across positions 2 and 3 of thymidine. An inhibitor of HIV-1 reverse transcriptase It has a role as an antimetabolite, an EC 2.7.7.49 (RNA-directed DNA polymerase) inhibitor and an antiviral agent. It is an organic molecular entity, a nucleoside analogue and a dihydrofuran. It is functionally related to a thymine.
A dideoxynucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV.
This compound is a Human Immunodeficiency Virus Nucleoside Analog Reverse Transcriptase Inhibitor. The mechanism of action of this compound is as a Nucleoside Reverse Transcriptase Inhibitor.
This compound is a first generation nucleoside analogue and reverse transcriptase inhibitor used in combination with other agents in the therapy of human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). This compound is an uncommon, but well established cause of clinically apparent acute and chronic liver injury.
This compound is a nucleoside reverse transcriptase inhibitor analog of thymidine. This compound has been recommended to undergo phase-out management due to its long-term, irreversible side-effects.
See also: Lamivudine; this compound (component of); this compound; lamivudine; nevirapine (component of); this compound; lamivudine (component of) ... View More ...
Properties
IUPAC Name |
1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XNKLLVCARDGLGL-JGVFFNPUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=CN(C(=O)NC1=O)C2C=CC(O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=CN(C(=O)NC1=O)[C@H]2C=C[C@H](O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12N2O4 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID1023819 | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
224.21 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
2',3'-didehydro-3'-deoxythymidine appears as white crystalline solid or powder. Odorless. (NTP, 1992), Solid | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
50 to 100 mg/mL at 70 °F (NTP, 1992), 5-10 g/100 mL at 21 °C, 30 mg/mL in propylene glycol at 23 °C, In water, 83 mg/mL at 23 °C, 4.05e+01 g/L | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
9.5X10-12 mm Hg at 25 °C /Estimated/ | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off white crystalline solid, Colorless granular solid from ethanol/benzene | |
CAS No. |
3056-17-5 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=3056-17-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Stavudine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0003056175 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | stavudine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759897 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1-((2R, 5S)-5-(hydroxymethyl)-2,5-dihydro-2-furanyl)-5-methyl-2,4(1H, 3H)-pyrimidinedione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | STAVUDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/BO9LE4QFZF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
318 to 320 °F (NTP, 1992), 159-160 °C, 165-166 °C, 159 - 160 °C | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Molecular and Cellular Mechanisms of Stavudine Action
Stavudine as a Nucleoside Analog of Thymidine
This compound is structurally similar to the naturally occurring nucleoside thymidine. nih.govwikipedia.org This structural mimicry is crucial for its mechanism of action as it allows this compound to be recognized and processed by cellular enzymes that normally handle thymidine. patsnap.com Specifically, this compound is a 2',3'-didehydro-3'-deoxythymidine, differing from thymidine by the absence of a hydroxyl group at the 3' carbon and the presence of a double bond between the 2' and 3' carbons of the deoxyribose ring. nih.govfda.govoncohemakey.com This structural modification is key to its function as a chain terminator. patsnap.comnih.gov
Intracellular Phosphorylation Pathways of this compound
For this compound to become antivirally active, it must undergo sequential phosphorylation within the host cell to form its active triphosphate metabolite, this compound triphosphate (d4T-TP). nih.govfda.govnih.gov This metabolic activation is a prerequisite for its inhibitory effects on HIV reverse transcriptase. nih.gov
Cellular Kinases Involved in Triphosphate Formation
The phosphorylation of this compound is carried out by several cellular kinases. The initial and often rate-limiting step involves the phosphorylation of this compound to this compound monophosphate (d4T-MP). oncohemakey.com This step is primarily mediated by cellular thymidine kinase 1 (TK1). oncohemakey.comnih.gov Subsequent phosphorylations convert d4T-MP to this compound diphosphate (d4T-DP) and then to the active this compound triphosphate (d4T-TP). These steps involve thymidylate kinase and nucleoside diphosphate kinase, respectively. oncohemakey.comresearchgate.net
Research findings indicate that while TK1 is a primary enzyme involved, it may not be the sole enzyme responsible for this compound's activation. nih.gov Studies have also explored the influence of prior exposure to other nucleoside analogs, such as zidovudine (AZT), on this compound phosphorylation. While some in vitro studies suggested potential competitive inhibition of phosphorylation by zidovudine, clinical studies have not consistently shown a significant difference in intracellular this compound triphosphate levels between zidovudine-naive and zidovudine-experienced patients. nih.govepividian.com However, the combination of zidovudine and this compound is generally not recommended due to the potential for competitive inhibition of intracellular phosphorylation. fda.goveuropa.eu
Competition with Endogenous Nucleoside Triphosphates
Once formed, this compound triphosphate (d4T-TP) acts by competing with the natural cellular substrate, deoxythymidine triphosphate (dTTP), for incorporation into the newly synthesized viral DNA by HIV-1 reverse transcriptase. nih.govfda.govfda.gov This competition is a key aspect of its inhibitory mechanism. The ratio of intracellular this compound triphosphate to endogenous deoxythymidine triphosphate is considered an important factor reflecting the direct competition at the level of reverse transcriptase. nih.govasm.org
Data from studies show the competitive nature of this interaction. For instance, the inhibition constant (Ki) of this compound triphosphate for HIV reverse transcriptase has been reported in the range of 0.0083 to 0.032 µM, indicating a high affinity for the enzyme's active site where dTTP would normally bind. nih.govfda.govfda.govfda.gov
Inhibition of HIV-1 Reverse Transcriptase Activity
The primary target of activated this compound is the HIV-1 reverse transcriptase enzyme. patsnap.comscielo.brnih.gov This enzyme is crucial for the virus to convert its single-stranded RNA genome into a double-stranded DNA copy, which is then integrated into the host cell's genome. wikipedia.orgasm.org this compound triphosphate inhibits this process through two main mechanisms. nih.govdrugbank.com
Competitive Inhibition Mechanisms
This compound triphosphate acts as a competitive inhibitor of HIV-1 reverse transcriptase by vying with the natural substrate, deoxythymidine triphosphate (dTTP), for binding to the enzyme's active site. nih.govfda.govnih.govfda.gov Due to its structural similarity to dTTP, d4T-TP can bind to the polymerase active site of reverse transcriptase. mdpi.com This competition reduces the availability of the active site for the natural substrate, thereby hindering the synthesis of viral DNA. patsnap.comasm.org
Detailed research findings, including kinetic studies, have characterized this competitive inhibition. The relatively low Ki values for d4T-TP against HIV-1 reverse transcriptase demonstrate its potency in competing with dTTP. nih.govfda.govfda.gov
DNA Chain Termination Mechanisms
The most critical aspect of this compound's action is its ability to cause DNA chain termination. patsnap.comnih.govnih.gov Once this compound triphosphate is incorporated into the growing viral DNA chain by reverse transcriptase, it prevents further elongation of the DNA strand. patsnap.comnih.govmdpi.com This occurs because this compound lacks a hydroxyl (OH) group at the 3' carbon position of its deoxyribose sugar. patsnap.comnih.govoncohemakey.com The 3'-OH group is essential for forming the phosphodiester bond with the next incoming nucleotide, a step required for DNA chain elongation. patsnap.comnih.govresearchgate.net The absence of this group in incorporated this compound results in an obligate chain termination event, effectively halting the synthesis of the viral DNA. nih.govoncohemakey.com
This premature termination of the viral DNA chain renders it incomplete and non-functional, preventing the integration of the viral genetic material into the host cell genome and thus inhibiting viral replication. patsnap.comwikipedia.org
Antiviral Efficacy and Viral Dynamics Research
In Vitro Antiviral Activity Studies
In vitro studies have been crucial in understanding the direct effects of stavudine on HIV-1 replication at the cellular level. These studies have investigated its inhibitory activity in different cell lines and primary cells, as well as the relationship between drug concentration and antiviral effect.
Inhibition of HIV-1 Replication in Cell Lines
This compound has demonstrated inhibitory activity against HIV-1 replication in various cell lines, including lymphoblastoid cell lines. fda.govfda.gov Studies have shown that this compound can inhibit HIV-1 vector virus titer in a single cycle of replication. asm.org The concentration required to inhibit HIV-1 replication by 50% (IC50 or EC50) in cell culture has been reported to range from 0.009 to 4 µM against laboratory and clinical isolates of HIV-1. fda.govfda.gov
Inhibition of HIV-1 Replication in Primary Cells (e.g., PBMCs, Monocytic Cells)
This compound's antiviral activity has also been evaluated in primary cells, such as peripheral blood mononuclear cells (PBMCs) and monocytic cells, which are key target cells for HIV-1 infection in vivo. fda.govfda.govplos.orgresearchgate.netresearchgate.net Studies have shown that this compound can potently inhibit HIV-1 replication in human PBMCs. asm.org The antiviral activity has been demonstrated against a range of clinical isolates of HIV-1 cultured in human PBMCs and primary monocytes. researchgate.net While effective in macrophages and PBMCs, studies have indicated that this compound may have reduced inhibitory activity in astrocytes compared to macrophages and PBMCs in vitro. plos.orgplos.org
Concentration-Dependent Antiviral Effects
The antiviral effect of this compound is concentration-dependent. In vitro studies assessing the concentration of this compound necessary to inhibit HIV-1 replication by 50% (IC50 or EC50) have shown a range of values depending on the specific cell type and HIV-1 isolate used. fda.govfda.gov For example, EC50 values against laboratory and clinical isolates of HIV-1 in cell culture ranged from 0.009 to 4 µM. fda.govfda.gov The antiviral efficacy is linked to the intracellular concentration of its active metabolite, this compound triphosphate (d4T-TP). nih.govnih.gov Studies have also investigated the interaction of this compound with other antiviral nucleoside analogs, showing synergistic interactions with some compounds like lamivudine (3TC) and additive to antagonistic activity with others like zidovudine. fda.govfda.govasm.org
In Vivo Antiviral Activity and Virologic Response
In vivo studies have evaluated the effectiveness of this compound in reducing viral load and improving immunological markers in individuals with HIV-1 infection.
Viral Load Reduction Studies
Clinical studies have demonstrated that this compound, particularly when used in combination with other antiretroviral agents, can lead to significant decreases in plasma viral load in patients with HIV-1 infection. i-base.infonih.gov Studies have shown that combinations including this compound can reduce viral load, often to undetectable levels. i-base.info In antiretroviral-naive patients, combinations of this compound with other nucleoside analogs like didanosine or lamivudine have been shown to effectively reduce viral loads. nih.gov Studies in surrogate models, such as the Hu-PBL-SCID mouse model, have also shown potent in vivo anti-HIV activity of this compound derivatives, exhibiting dose-dependent effects. asm.orgasm.org
CD4+ T-Lymphocyte Count Dynamics in Response to this compound
Here is a summary of some in vitro antiviral activity data:
| Cell Type | HIV-1 Isolate Type | EC50 Range (µM) | Reference |
| Peripheral Blood Mononuclear Cells | Laboratory and Clinical | 0.009 - 4 | fda.govfda.gov |
| Monocytic Cells | Laboratory and Clinical | 0.009 - 4 | fda.govfda.gov |
| Lymphoblastoid Cell Lines | Laboratory and Clinical | 0.009 - 4 | fda.govfda.gov |
| MOLT-4/IIIB (HIV-1 infected) | IIIB | 2.2 | researchgate.net |
| MOLT-4 (uninfected) | N/A | 59.8 | researchgate.net |
| MT-4/1 cell line | Various strains | Dose-dependent | crie.ru |
| MT-4/2 cell line | Various strains | Dose-dependent | crie.ru |
Here is a summary of some in vivo virologic and immunologic response data:
| Study Type | Patient Population | Key Virologic Finding | Key Immunologic Finding | Reference |
| Clinical Studies (Combination Therapy) | Antiretroviral-naive HIV-1 infected patients | Reduced viral loads | Increased CD4 cell counts | nih.gov |
| Clinical Studies | Patients with advanced HIV-1 infection | Reduced viral load (often undetectable) | Increased CD4 cells | i-base.info |
| Clinical Studies | This compound-treated groups | Decreases in serum p24 antigen | Significant increases in mean CD4 cell counts | nih.gov |
| Hu-PBL-SCID mouse model (this compound derivative) | Mice infected with NRTI-resistant clinical HIV-1 isolate | Potent in vivo anti-HIV activity (dose-dependent) | Not specified | asm.orgasm.org |
Antiviral Synergy and Antagonism in Combination Therapies
Combination antiretroviral therapy (ART) is the standard of care for HIV-1 infection, aiming to achieve maximal viral suppression and prevent the emergence of drug resistance. Understanding the interactions between different antiretroviral agents is crucial for designing effective and well-tolerated regimens. Studies have evaluated the interactions of this compound with other drugs, both within the NRTI class and with other classes of antiretroviral agents.
Interactions with Other Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
In vitro studies have explored the interactions between this compound and other NRTIs, revealing a range of outcomes from synergy to antagonism. This compound in combination with zidovudine (AZT) has shown additive to antagonistic activity in cell culture. fda.govfda.gov This in vitro antagonism between zidovudine and this compound was also observed in a clinical setting, where HIV-1-infected patients responded better to this compound monotherapy than to the combination of this compound and zidovudine. nih.gov This antagonism is thought to be related to competitive inhibition of intracellular phosphorylation. fda.govnatap.org
Conversely, this compound has exhibited additive to synergistic anti-HIV-1 activity when combined with other NRTIs such as abacavir, didanosine (ddI), tenofovir, or zalcitabine (ddC) in vitro. fda.govfda.gov
Data on the interaction between this compound and other NRTIs is summarized in the table below:
| Combination | In vitro Interaction | Clinical Observation (if available) | Mechanism (if known) |
| This compound + Zidovudine | Additive to Antagonistic fda.govfda.gov | Less effective than monotherapy nih.gov | Competitive inhibition of intracellular phosphorylation fda.govnatap.org |
| This compound + Abacavir | Additive to Synergistic fda.govfda.gov | Not specified in search results | Not specified in search results |
| This compound + Didanosine | Additive to Synergistic fda.govfda.gov | Caution advised in pregnancy due to risk of lactic acidosis fda.govfda.govfda.gov | Not specified in search results |
| This compound + Tenofovir | Additive to Synergistic fda.govfda.gov | Not specified in search results | Not specified in search results |
| This compound + Zalcitabine | Additive to Synergistic fda.govfda.gov | Not specified in search results | Not specified in search results |
Interactions with Other Antiretroviral Classes
Research has also investigated the interactions of this compound with antiretroviral drugs from other classes, including non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs).
In cell culture, nevirapine, an NNRTI, demonstrated additive to synergistic activity against HIV-1 in combination regimens that included this compound and other NRTIs. fda.gov Studies evaluating combinations of tenofovir and emtricitabine with a third agent from major drug classes like NNRTIs or PIs have generally shown additive to synergistic anti-HIV-1 activity in vitro. asm.org While these studies may not have focused specifically on this compound in triple combinations, they suggest that NRTIs, as a class, can exhibit favorable interactions with NNRTIs and PIs.
Specific drug interaction studies with efavirenz and NRTIs other than lamivudine and zidovudine have not always been performed, but clinically significant interactions are not typically expected because NRTIs are metabolized differently than efavirenz, reducing the likelihood of competition for metabolic enzymes and elimination pathways. fda.gov
Combinations involving CCR5 antagonists, a class of entry inhibitors, have also been studied. For instance, a novel CCR5 antagonist, TAK-220, showed interactions ranging from low-level antagonism at low inhibitory concentrations to synergy at higher inhibitory concentrations when combined with reverse transcriptase and protease inhibitors. asm.org Another CCR5 antagonist, SCH-C, demonstrated strong synergy with representative drugs from all classes of currently used antiretroviral agents, including NRTIs. nih.gov
While comprehensive data specifically detailing this compound's interactions with every drug in other antiretroviral classes is extensive and varied, the general trend in vitro studies suggests that combinations of NRTIs with NNRTIs, PIs, and entry inhibitors can result in additive to synergistic antiviral effects. fda.govasm.orgasm.orgnih.gov
| Combination Class (this compound +) | Observed Interaction (In vitro) |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | Additive to Synergistic (e.g., with Nevirapine) fda.govasm.org |
| Protease Inhibitors (PIs) | Additive to Synergistic (in combinations with other NRTIs) asm.org |
| Entry Inhibitors (e.g., CCR5 antagonists) | Additive to Synergistic asm.orgnih.gov |
Mechanisms of Hiv-1 Resistance to Stavudine
Selection of Stavudine-Resistant HIV-1 Isolates In Vitro
In vitro selection experiments have been instrumental in understanding the emergence of HIV-1 resistance to this compound and predicting potential resistance pathways observed in vivo asm.orgasm.org. These studies involve culturing HIV-1 isolates in the presence of increasing concentrations of this compound over time. Under this selective drug pressure, viral variants with mutations that confer reduced susceptibility to this compound are favored and become the dominant viral population asm.orgplos.org. In vitro selection experiments have demonstrated that the kinetics of acquiring resistance mutations can differ between HIV-1 subtypes, suggesting potential subtype-specific resistance pathways plos.org.
Phenotypic and Genotypic Characterization of Resistance
Resistance to this compound is characterized both phenotypically and genotypically nih.govnih.govoup.com. Phenotypic characterization involves measuring the reduction in the virus's susceptibility to this compound in cell culture, typically expressed as a fold change in the half-maximal effective concentration (EC50) or half-maximal inhibitory concentration (IC50) compared to a wild-type reference strain nih.govnih.goviasusa.org. Genotypic characterization involves sequencing the reverse transcriptase gene to identify specific mutations associated with reduced this compound susceptibility nih.govnih.govstanford.edu.
Studies evaluating HIV-1 isolates from patients treated with this compound have shown varying degrees of phenotypic resistance. Some studies reported modest mean reductions in this compound susceptibility, often less than twofold or fourfold, even after prolonged treatment nih.govnih.govoup.com. For example, one study found a mean fold change in this compound susceptibility of 1.9 in isolates from patients treated with didanosine and this compound nih.gov. Another study on patients receiving prolonged this compound therapy identified only a small number of isolates with decreased this compound sensitivity (ED50s < 4-fold higher than pretreatment isolates) nih.govoup.com.
Genotypic analysis of these isolates often reveals the presence of multiple mutations in the reverse transcriptase gene nih.govoup.com. However, in some cases, a clear genetic basis directly accounting for the observed changes in this compound susceptibility was not immediately identified, suggesting complex interactions between mutations or other resistance mechanisms nih.govoup.com.
Identification of Reverse Transcriptase Gene Mutations
Resistance to NRTIs like this compound is primarily mediated by the acquisition of specific mutations in the HIV-1 reverse transcriptase gene. These mutations can affect the enzyme's ability to incorporate the this compound triphosphate analog or enhance its ability to remove the incorporated analog from the growing viral DNA chain nih.govtandfonline.com. The latter mechanism, known as phosphorolytic removal or primer unblocking, is particularly relevant for resistance to thymidine analogs like this compound and zidovudine nih.govtandfonline.com.
Mutations associated with this compound resistance often overlap with those selected by other NRTIs, particularly zidovudine.
Zidovudine-Resistance Associated Mutations (e.g., M41L, D67N, K70R, L210W, T215Y/F, K219Q/E) and Cross-Resistance to this compound
A key group of mutations associated with resistance to thymidine analogs, including both zidovudine (AZT) and this compound (d4T), are known as Thymidine Analogue Mutations (TAMs) plos.orgnih.govasm.org. These mutations include M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E nih.govasm.orgiasusa.org. TAMs are selected for during treatment with either zidovudine or this compound plos.orgnih.govasm.orgiasusa.org.
TAMs confer cross-resistance to this compound and a varying degree of cross-resistance to most other NRTIs stanford.edunih.govtandfonline.comasm.orgiasusa.org. The mechanism by which TAMs mediate resistance involves enhancing the ATP-dependent phosphorolytic removal of the incorporated nucleoside analog from the 3' end of the viral DNA primer, effectively "unblocking" the primer and allowing DNA synthesis to continue nih.govtandfonline.comasm.org.
Two main pathways for the accumulation of TAMs have been described:
TAM-1 pathway: Typically involves mutations at positions M41L, L210W, and T215Y plos.orgnih.govasm.orgiasusa.org.
TAM-2 pathway: Typically involves mutations at positions D67N, K70R, T215F, and K219Q/E plos.orgnih.govasm.orgiasusa.org.
The accumulation of multiple TAMs generally leads to increasing levels of resistance to this compound and other NRTIs tandfonline.comiasusa.orgiasusa.org. Prior exposure to zidovudine and the presence of TAMs, especially at codon 215 (T215Y/F), have been shown to negatively impact the virological response to subsequent this compound-containing regimens stanford.edu.
Multi-Nucleoside Resistance Associated Mutations (e.g., Q151M)
Another important mutation associated with resistance to this compound, particularly in the context of multi-nucleoside resistance, is Q151M nih.govasm.orgplos.org. The Q151M mutation is often considered a marker for a broader multi-nucleoside resistance profile asm.org. This resistance pattern frequently involves the accumulation of the Q151M mutation along with other specific mutations, including A62V, V75I, F77L, and F116Y, forming the Q151M complex nih.govasm.orgstanford.educsic.es.
The Q151M complex confers high-level resistance to a range of NRTIs, including zidovudine, didanosine, zalcitabine, and this compound nih.govasm.orgstanford.educsic.es. While the full complex leads to high-level resistance, the Q151M mutation alone can confer low-level resistance to these drugs asm.org. Unlike the TAM-mediated resistance which involves phosphorolytic removal, the mechanism of resistance conferred by Q151M appears to be related to reduced binding of the NRTI triphosphate to the reverse transcriptase enzyme, independent of ATP asm.org. The Q151M mutation has been specifically associated with this compound use in some clinical settings plos.org. Importantly, viruses with the Q151M mutation generally retain susceptibility to tenofovir plos.orgiasusa.orgnih.govplos.orgstanford.edu.
Viral Fitness and Replicative Capacity of Resistant Strains
The acquisition of drug resistance mutations in the HIV-1 reverse transcriptase gene can impact the virus's ability to replicate efficiently in the absence of drug pressure, a property referred to as viral fitness or replicative capacity nih.govnih.govasm.orgoup.com. While resistance mutations allow the virus to survive and replicate in the presence of antiretroviral drugs, they can sometimes come at a cost to the intrinsic efficiency of the viral replication machinery nih.govnih.gov.
The degree to which resistance mutations impair viral fitness varies depending on the specific mutation or combination of mutations nih.govnih.gov. Mutations like the TAMs and those within the Q151M complex have been shown to affect viral fitness nih.gov. In some instances, the reduced replicative capacity of drug-resistant strains may contribute to a partial virological control or immunological stability despite ongoing low-level viral replication in treated patients with limited treatment options nih.govoup.com.
Compound Names and PubChem CIDs:
| Compound Name | PubChem CID |
| This compound | 18283 |
Data Table Example (Illustrative based on search findings):
| Mutation(s) Present | Associated Resistance | Mechanism | Fold Change in Susceptibility (Example Range) | Impact on Viral Fitness (General) |
| TAMs (e.g., M41L, T215Y) | This compound, Zidovudine, other NRTIs | Enhanced phosphorolytic removal (Primer Unblocking) nih.govtandfonline.com | Variable, increases with accumulation iasusa.orgiasusa.org | Often reduced, can be compensated nih.gov |
| Q151M Complex (with A62V, V75I, F77L, F116Y) | Multi-nucleoside resistance (this compound, Zidovudine, ddI, ddC, ABC) nih.govasm.orgstanford.edu | Reduced binding of NRTI-TP asm.org | High-level asm.orgstanford.edu | Often reduced nih.gov |
| Q151M alone | Low-level multi-nucleoside resistance asm.org | Reduced binding of NRTI-TP asm.org | Low-level asm.org | Variable |
Note: The specific fold change in susceptibility can vary widely depending on the specific combination and number of mutations, as well as the assay used. The "Impact on Viral Fitness" is a general observation, and specific mutations or combinations can have different effects.
Pharmacology and Metabolism Research Mechanistic Aspects
Intracellular Pharmacokinetics and Activation of Stavudine
For this compound to exert its antiviral effect, it must first enter target cells and be converted to its active triphosphate form. patsnap.comdrugbank.com
Cellular Uptake Mechanisms
The parent compound, this compound, enters cells, including peripheral blood mononuclear cells, via passive diffusion. oncohemakey.com Research involving liposomes has explored methods to enhance the cellular uptake of this compound, particularly in macrophages. Studies with liposomes containing dipalmitoyl phosphatidylcholine (DPPC) showed maximal this compound uptake. researchgate.net The presence of sphingomyelin decreased uptake, while a negative charge on the liposome bilayer enhanced it compared to a positive charge. researchgate.net Polysaccharide coatings on lipid nanoparticles have also been investigated as a means to improve cellular uptake, potentially through specific receptors involved in tissue addressing and transport mechanisms. omicsonline.org
Pathways of Intracellular Phosphorylation to this compound Triphosphate
This compound is a prodrug that requires intracellular phosphorylation by cellular kinases to become the active metabolite, this compound triphosphate (d4T-TP). patsnap.comdrugbank.comoncohemakey.comwikipedia.orgfda.govontosight.aipatsnap.com This sequential phosphorylation involves the enzymes thymidine kinase, thymidylate kinase, and nucleoside diphosphate kinase. researchgate.netresearchgate.net The initial phosphorylation step, catalyzed by thymidine kinase, appears to be the rate-limiting step in the activation of this compound. oncohemakey.com Unlike zidovudine, this compound does not result in the accumulation of the monophosphate form. oncohemakey.com The mono-, di-, and triphosphate forms of this compound are present in an approximately 1:1:1 ratio intracellularly, and increasing the extracellular concentration of this compound leads to proportional increases in the intracellular concentration of the active triphosphate form. oncohemakey.com this compound triphosphate inhibits HIV-1 reverse transcriptase by competing with the natural substrate, thymidine triphosphate, and by causing DNA chain termination due to the absence of a 3'-hydroxyl group necessary for DNA elongation. patsnap.comdrugbank.comoncohemakey.comwikipedia.orgfda.govontosight.aipatsnap.comeuropa.eunih.govmims.com this compound triphosphate also inhibits cellular DNA polymerases β and γ, which can lead to a reduction in mitochondrial DNA synthesis. fda.govpatsnap.comnih.gov
The intracellular half-life of this compound triphosphate has been reported as approximately 3.5 hours in CEM T-cells and peripheral blood mononuclear cells. europa.eu
Enzymatic Pathways of this compound Metabolism and Elimination
This compound is eliminated from the body through a combination of metabolic processes and renal excretion. nih.govasm.org
Glucuronidation Pathways
Glucuronidation is one of the metabolic pathways identified for this compound. wikipedia.orgontosight.ai This process involves the conjugation of this compound or its oxidized metabolite with glucuronic acid, forming glucuronide conjugates. ontosight.aieuropa.eueuropa.euvulcanchem.comcymitquimica.com Enzymes of the UDP-glucuronosyltransferase (UGT) family are involved in this phase II metabolism of this compound. researchgate.netresearchgate.net Glucuronidation typically increases the water solubility of compounds, facilitating their excretion. wikipedia.org
Impact of Hepatic Metabolism on this compound Clearance
While renal excretion is a primary route of elimination, hepatic metabolism also contributes to this compound clearance. nih.govasm.org The clearance of this compound is minimally affected by hepatic metabolism. wikipedia.org Metabolites, including oxidized this compound and its glucuronide conjugates, have been identified in plasma and urine. europa.eueuropa.eu Studies in patients with hepatic impairment secondary to cirrhosis showed that this compound pharmacokinetics were similar to those in patients with normal hepatic function, suggesting that hepatic impairment does not substantially alter this compound clearance. fda.govasm.org this compound does not significantly inhibit major cytochrome P450 isoforms, making clinically significant drug interactions through this pathway unlikely. fda.goveuropa.eu
Data Tables
| Pharmacokinetic Parameter (Adults) | Mean ± SD | Units | Source |
| Oral Bioavailability (F) | 86.4 ± 18.2 | % | fda.gov |
| Volume of Distribution | 46 ± 21 | L | drugbank.comfda.gov |
| Protein Binding | Negligible | drugbank.comfda.goveuropa.eufda.govfda.govfda.gov | |
| Terminal Elimination Half-life | 0.8-1.5 | hours | drugbank.comwikipedia.org |
| Renal Clearance (Healthy Subjects) | ~272 | mL/min | drugbank.comeuropa.eueuropa.eu |
| Renal Clearance (HIV-infected) | 237 ± 98 | mL/min | europa.eueuropa.eu |
| Overall Renal Clearance | ~40 | % of dose | fda.govontosight.aieuropa.euasm.orgfda.govwho.intfda.gov |
| Intracellular Half-life (d4T-TP) | ~3.5 | hours | europa.eu |
Mechanisms of Stavudine-associated Toxicities and Adverse Events
Mitochondrial Toxicity Pathogenesis
Stavudine's impact on mitochondrial function is largely attributed to its interference with mitochondrial DNA (mtDNA) replication and subsequent effects on cellular energy production. oup.comoup.com
Inhibition of DNA Polymerase Gamma Leading to Mitochondrial DNA Depletion
Mitochondrial DNA is replicated by DNA polymerase gamma (Pol γ), an enzyme encoded by the nuclear gene POLG. oup.com this compound, after being anabolized to its active triphosphate form (d4TTP), acts as a potent inhibitor and alternative substrate for Pol γ. nih.govnih.govasm.org This competitive inhibition and incorporation into the growing mtDNA chain by d4TTP leads to premature chain termination and a significant reduction in mtDNA copy number within cells. oup.comoup.comnatap.org
Studies have shown that this compound can induce a higher degree of mtDNA depletion compared to other NRTIs like zidovudine and abacavir. natap.org This depletion of mtDNA is a key factor in the pathogenesis of this compound-associated mitochondrial toxicity. natap.org
Data from studies comparing mtDNA levels in different tissues of patients on NRTI therapy highlight the impact of this compound. For instance, one study observed that NRTI treatment was associated with a significant reduction in adipocyte mtDNA copies per cell, with individuals receiving this compound showing a mean mtDNA depletion of 87.1% compared to 52.1% in those receiving zidovudine. natap.org
Cellular and Tissue-Specific Mitochondrial Impairment
The consequences of mtDNA depletion and Pol γ inhibition by this compound manifest as impaired mitochondrial function, affecting various tissues and cell types to different extents. oup.commdpi.com Tissues with high energy demands, such as muscle, liver, and adipose tissue, appear particularly vulnerable to this compound-induced mitochondrial toxicity. oup.comnatap.orgmdpi.com
Mitochondrial dysfunction can lead to a decrease in the activity of the electron transport chain (ETC), which is crucial for oxidative phosphorylation and ATP production. mdpi.com This energy deficiency can contribute to the observed clinical toxicities. Studies have shown impaired mitochondrial enzyme activities, such as complex I, in patients with this compound-associated lipoatrophy. natap.org
Research in different cell types, including adipocytes and hepatocytes, has demonstrated this compound's ability to induce mitochondrial dysfunction. nih.govnih.govnih.gov While severe mtDNA depletion has been observed in both white and brown adipocytes exposed to this compound, the specific mechanisms of toxicity can vary depending on the cell type. nih.gov Some studies suggest that this compound might also affect mitochondrial function through mechanisms independent of mtDNA depletion, such as altering fatty acid oxidation enzymes or cofactors. nih.govnih.govresearchgate.net
Genetic Predisposition to Mitochondrial Toxicity (e.g., DNA Polymerase Gamma Mutations like R964C)
Genetic factors can influence an individual's susceptibility to this compound-induced mitochondrial toxicity. Variations in the nuclear gene encoding DNA polymerase gamma (POLG) have been implicated in predisposing patients to adverse events. oup.comfrontiersin.org
A specific mutation in POLG, resulting in an arginine to cysteine substitution at codon 964 (R964C), has been linked to increased susceptibility to this compound-mediated mitochondrial toxicity and severe lactic acidosis. oup.comnih.govnih.govasm.org Studies utilizing pre-steady-state kinetics have shown that the R964C mutation in Pol γ leads to a decreased efficiency in incorporating the natural substrate (dTTP) and a reduced ability to discriminate against the incorporation of this compound's active metabolite (d4TTP). nih.govnih.govasm.org This altered discrimination increases the likelihood of d4TTP incorporation into mtDNA, exacerbating the mtDNA depletion and subsequent mitochondrial dysfunction. nih.govnih.govasm.org
Research on lymphoblastoid cell lines harboring the R964C mutation has demonstrated significantly reduced mtDNA levels when cultured with this compound compared to cell lines with wild-type Pol γ. oup.com This provides a mechanistic basis for the observed genetic predisposition to this compound toxicity in individuals carrying this mutation. nih.govnih.govasm.org
Lactic Acidosis and Hepatic Steatosis Mechanisms
Lactic acidosis and hepatic steatosis are serious adverse events associated with this compound therapy, both linked to mitochondrial dysfunction, particularly in hepatocytes. europa.eunatap.orgmdpi.comacpjournals.orgnih.gov
Mitochondrial Dysfunction in Hepatocytes
Hepatocytes, the primary cells of the liver, are rich in mitochondria and highly dependent on their function for various metabolic processes, including energy production and lipid metabolism. mdpi.commedcraveonline.com this compound-induced mitochondrial toxicity in hepatocytes plays a central role in the development of hepatic steatosis and lactic acidosis. natap.orgacpjournals.orgnih.govjwatch.org
The inhibition of Pol γ and subsequent mtDNA depletion in hepatocytes impair the function of the mitochondrial respiratory chain, which is essential for oxidative phosphorylation. mdpi.com This leads to decreased ATP production and can disrupt cellular energy homeostasis. mdpi.com
Studies have shown that this compound can cause mtDNA depletion in cultured hepatocytes. nih.gov Liver biopsies from patients treated with this compound have also revealed decreased hepatic mtDNA content. nih.gov This mitochondrial impairment in hepatocytes contributes to the metabolic derangements observed in lactic acidosis and hepatic steatosis. natap.orgmdpi.comjwatch.org
Impairment of Lactic Acid and Fatty Acid Metabolism
Mitochondrial dysfunction in hepatocytes directly impacts the metabolism of both lactic acid and fatty acids. Under normal conditions, the mitochondria are involved in the clearance of lactate through the Cori cycle and the efficient oxidation of fatty acids to produce energy. mdpi.comjwatch.org
Impaired mitochondrial function due to this compound toxicity can lead to a reduced capacity of hepatocytes to metabolize lactic acid. natap.orgmdpi.comjwatch.org This can result in the accumulation of lactate in the bloodstream, leading to hyperlactatemia and, in severe cases, lactic acidosis. europa.eunatap.orgacpjournals.orgnih.govjwatch.org
Furthermore, mitochondrial dysfunction impairs fatty acid oxidation (FAO), a process primarily carried out in the mitochondria to break down fatty acids for energy. mdpi.comnih.govmdpi.com When FAO is inhibited, there is an increased esterification of fatty acids into triglycerides, leading to the accumulation of fat droplets within hepatocytes, a condition known as hepatic steatosis. mdpi.comnih.govmdpi.com While mtDNA depletion is considered a primary mechanism for impaired FAO and steatosis, some research suggests that high concentrations of this compound might directly inhibit FAO in hepatocytes without necessarily causing mtDNA depletion. nih.govnih.govresearchgate.net This highlights the complexity of this compound's effects on hepatic lipid metabolism.
The combination of impaired lactate clearance and reduced fatty acid oxidation due to mitochondrial dysfunction in hepatocytes contributes significantly to the pathogenesis of lactic acidosis and hepatic steatosis observed in patients treated with this compound. natap.orgmdpi.comjwatch.org
Association with Severe Hepatomegaly
Severe hepatomegaly, often accompanied by hepatic steatosis (fatty liver), is a serious adverse event reported with the use of nucleoside analogues, including this compound. europa.eufda.gov Cases of lactic acidosis, sometimes fatal, have been reported in less than 1% of patients taking this compound in combination with other antiretrovirals, and this is usually associated with severe hepatomegaly and hepatic steatosis. europa.eu this compound can cause a dangerous enlargement of the liver accompanied by the buildup of fat in liver cells. patsnap.com The exact mechanisms leading specifically to severe hepatomegaly with this compound are intertwined with the broader picture of mitochondrial toxicity and lactic acidosis. Treatment with this compound should be discontinued if there is progressive hepatomegaly or rapidly elevating aminotransferase levels. europa.eu Caution is advised when administering this compound to patients with pre-existing hepatomegaly, hepatitis, or other known risk factors for liver disease and hepatic steatosis. europa.eu
Lipodystrophy Mechanisms, Particularly Lipoatrophy
Lipodystrophy, characterized by abnormal changes in body fat distribution, is a notable complication associated with older antiretroviral drugs, particularly this compound. aidsmap.com Lipoatrophy, the loss of subcutaneous fat, is a key component of this syndrome linked to this compound use. aidsmap.comhiv.gov
Mitochondrial Toxicity in Adipocytes
A primary mechanism underlying this compound-associated lipoatrophy is mitochondrial toxicity. europa.euaidsmap.comhiv.gov this compound, like other NRTIs, can inhibit mitochondrial DNA polymerase gamma, an enzyme crucial for mitochondrial DNA (mtDNA) replication. hiv.govoup.comnih.gov This inhibition leads to the depletion of mtDNA in various tissues, including adipocytes (fat cells). hiv.govoup.comnatap.org Research has shown that the loss of fat may be caused when mitochondria are damaged or decline in number as a result of older medications like this compound. aidsmap.com Studies in adipocytes have demonstrated that this compound induces severe mitochondrial DNA depletion. nih.gov This mitochondrial dysfunction in adipocytes results in decreased lipogenesis (fat production) and an increase in lipoapoptotic mediators, contributing to the loss of subcutaneous fat. nih.gov
Genetic Markers Associated with Lipoatrophy (e.g., HLA-B*4001)
Genetic factors can influence an individual's susceptibility to this compound-associated lipodystrophy. The human leukocyte antigen (HLA) allele HLA-B4001 has been identified as a strong genetic risk factor for this compound-associated lipodystrophy in HIV-infected patients. oup.comnih.govoup.comnih.gov Studies have shown a significant association between the presence of HLA-B4001 and the development of moderate to severe lipodystrophy in patients receiving this compound. oup.com This genetic factor appears to be a stronger predictor than the duration of this compound treatment in some populations. oup.comnih.gov HLA-B*4001 has demonstrated high specificity and a positive predictive value for lipodystrophy. nih.govoup.comnih.gov
Genetic Association Data for HLA-B*4001 and this compound-Associated Lipodystrophy
| Genetic Marker | Association with Lipodystrophy | Odds Ratio (95% CI) | P-value | Specificity | Positive Predictive Value |
| HLA-B*4001 | Strong genetic risk factor | 14.05 (2.57–76.59) nih.gov | 0.002 nih.gov | 95.8% nih.govoup.comnih.gov | 88.9% nih.govoup.comnih.gov |
Note: Data primarily from a study in Thailand. oup.comnih.govoup.com
Peripheral Neuropathy Pathogenesis
Peripheral neuropathy, a common side effect of this compound, is characterized by problems with the peripheral nerves. wikipedia.org It is often dose-related and occurs more frequently in patients with advanced HIV disease or a prior history of peripheral neuropathy. hiv.gov
Mitochondrial Impairment in Axons and Schwann Cells
Mitochondrial toxicity is considered a potential underlying mechanism for the polyneuropathy associated with this compound therapy. europa.eueuropa.eu this compound's ability to inhibit mitochondrial DNA polymerase gamma can lead to mitochondrial dysfunction in nerve cells, including axons and Schwann cells. hiv.govnih.gov Damage to the mitochondria caused by NRTIs like this compound can lead to symptoms such as damage to the peripheral nerves. aidsmap.com Studies have shown morphological changes in mitochondria, including lack of crista definition and homogenized matrix, in myelinated axons, unmyelinated axons, and Schwann's cells in cases of nucleoside analog-associated neuromyopathy, suggesting mitochondrial dysfunction as a common cause. bmj.combmj.com The pathophysiology of HIV-associated sensory neuropathy in patients on this compound may involve damage to the mitochondria of neurons and axons via damage to mitochondrial DNA. researchgate.net
Dose-Related Mechanisms and Advanced HIV Disease Association
The incidence and severity of some this compound-associated toxicities, particularly peripheral neuropathy and lipodystrophy (specifically lipoatrophy), are dose-related. hiv.govwikipedia.orgplos.org Studies have shown that patients receiving higher doses of this compound (e.g., 40 mg twice daily) experience higher rates of toxicity and a shorter time to toxicity diagnosis compared to those receiving lower doses (e.g., 30 mg twice daily). plos.org
Advanced HIV disease is also associated with an increased frequency of peripheral neuropathy in patients treated with this compound. hiv.govwikipedia.orgfda.govfda.gov This suggests that the underlying health status and immune compromise in advanced disease may contribute to the susceptibility to this compound-induced nerve damage.
Resolution of Symptoms with Prompt Discontinuation
Prompt discontinuation of this compound therapy upon the development of symptoms can lead to the resolution or improvement of certain adverse effects, such as peripheral neuropathy and lipodystrophy. hiv.govwikipedia.orgfda.goveuropa.eu For peripheral neuropathy, symptoms may resolve after dose reduction or interruption of the drug. europa.eu In some cases of lipodystrophy, improvements or resolution have been reported after discontinuing this compound. hiv.govhiv.gov However, it's important to note that in some instances, symptoms, particularly motor weakness associated with lactic acidosis, may continue or worsen even after discontinuation. fda.govfda.govdrugs.com
Hepatic Toxicity Mechanisms (Beyond Lactic Acidosis)
Beyond its contribution to lactic acidosis, this compound is a recognized cause of clinically apparent acute and chronic liver injury. nih.govnih.gov
Elevated Liver Enzymes and Transaminases
Elevations in liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), are observed in patients taking this compound. nih.govdrugs.comajronline.org Mild and transient elevations can occur in a significant proportion of patients, while more pronounced elevations (above 5 times the upper limit of normal) are less common but still reported. nih.gov this compound's effects on mitochondrial toxicity are considered partially responsible for these elevated liver enzyme levels. omicsonline.org
Hepatitis and Liver Failure Pathophysiology
This compound can lead to severe hepatic adverse events, including hepatitis and liver failure, which can be fatal. fda.goveuropa.eu The pathophysiology involves mitochondrial injury in hepatocytes, leading to impaired metabolism of lactic acid and free fatty acids, as well as compromised hepatic synthetic and excretory functions. nih.gov Liver histology in the early stages often shows marked microvesicular steatosis (fat accumulation) with minimal hepatocyte injury. nih.gov As the injury progresses, cholestasis may develop, and the fatty change can evolve into a macrovesicular pattern. nih.gov Late-stage changes may include ballooning cell degeneration, Mallory bodies, and fibrosis. nih.gov
Risk Factors for Hepatic Decompensation (e.g., Co-infection with Hepatitis B or C)
Several risk factors increase the likelihood of hepatic decompensation and severe liver injury in patients receiving this compound. Preexisting liver dysfunction, including chronic active hepatitis, is a significant risk factor. fda.govfda.gov Co-infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) substantially increases the risk of liver injury and hepatic decompensation in HIV-infected patients on antiretroviral therapy, including this compound. nih.govomicsonline.orgnih.govmedscape.comoup.comscirp.org These co-infections can accelerate liver injury progression. omicsonline.org Other factors associated with increased risk include female sex, older age, obesity, alcohol use, and concurrent therapy with other potentially hepatotoxic drugs, such as didanosine, ribavirin, and tenofovir. nih.govfda.govpediatriconcall.com
Pancreatitis Mechanisms
Pancreatitis is another serious adverse event associated with this compound therapy. hiv.govwikipedia.orgfda.govajronline.org While the exact mechanism of NRTI-induced pancreatic inflammation is not fully clear, it is believed to be linked to mitochondrial toxicity. ajronline.orgnih.gov The clinical manifestations of NRTI-induced mitochondrial toxicity, including pancreatitis, resemble those of inherited mitochondrial diseases. nih.gov Inhibition of mitochondrial DNA polymerase γ, adenylate kinase, and the adenosine diphosphate/adenosine triphosphate translocator can contribute to mitochondrial dysfunction and cellular toxicity in the pancreas. nih.gov The incidence of pancreatitis is higher when this compound is used in combination with other nucleoside analogs or cotrimoxazole. nih.gov Pancreatitis typically develops several months after initiating this compound therapy. ajronline.orgnih.gov
Data Table: Selected this compound-Associated Toxicities and Associated Factors
| Toxicity | Dose-Related? | Association with Advanced HIV Disease | Potential Mechanism | Resolution with Discontinuation | Risk Factors (Beyond this compound) |
| Peripheral Neuropathy | Yes hiv.govwikipedia.orgplos.org | Yes hiv.govwikipedia.orgfda.govfda.gov | Mitochondrial Toxicity hiv.goveuropa.euukzn.ac.zaru.nl | Yes (often) hiv.govwikipedia.orgfda.goveuropa.eu | History of neuropathy, concurrent neurotoxic drugs (e.g., didanosine) hiv.govwikipedia.orgfda.govfda.gov |
| Lipoatrophy | Yes wikipedia.orgplos.org | Not explicitly stated in sources | Mitochondrial Toxicity hiv.goveuropa.euukzn.ac.zaru.nl | Yes (sometimes) hiv.govhiv.gov | Not explicitly stated in sources |
| Elevated Liver Enzymes | Partially omicsonline.org | Not explicitly stated in sources | Mitochondrial Toxicity omicsonline.org | Yes (often transient) nih.gov | HBV/HCV co-infection, alcohol use, older age, obesity nih.govomicsonline.orgoup.comscirp.org |
| Hepatitis/Liver Failure | Indirectly (via dose-related toxicity) | Not explicitly stated in sources | Mitochondrial Toxicity europa.euukzn.ac.zaru.nlnih.gov | Yes (potential, especially if caught early) nih.gov | Preexisting liver dysfunction, HBV/HCV co-infection, alcohol use, older age, obesity, concomitant hepatotoxic drugs nih.govfda.govomicsonline.orgfda.govnih.govmedscape.comoup.comscirp.org |
| Pancreatitis | Not explicitly stated in sources | Yes (more advanced disease) ajronline.org | Mitochondrial Toxicity (suspected) ajronline.orgnih.gov | Not explicitly stated in sources | Concomitant nucleoside analogs (e.g., didanosine), cotrimoxazole fda.govnih.gov |
Genotoxicity and Carcinogenicity Studies
This compound's genotoxic and carcinogenic potential has been investigated through various in vitro and in vivo studies. The incorporation of NRTIs like this compound into nuclear DNA has been proposed as a mechanism leading to genotoxic and mutagenic effects. researchgate.net
In Vitro Genotoxic Effects (e.g., in human lymphocytes, mouse fibroblasts)
In vitro studies have shown this compound to produce positive results in assays assessing clastogenesis and cell transformation. This compound elevated the frequency of chromosome aberrations in human lymphocytes at concentrations ranging from 25 to 250 µg/mL, without metabolic activation. fda.govnih.govfda.gov It also increased the frequency of transformed foci in mouse fibroblast cells at concentrations from 25 to 2500 µg/mL, both with and without metabolic activation. fda.govnih.govfda.gov Oxidative stress mediated DNA damage has been suggested as a mechanism for its mutagenic and clastogenic effects in human lymphocytes and mouse fibroblast cells. cabidigitallibrary.org
In Vivo Genotoxic Effects (e.g., chromosomal aberrations)
In addition to in vitro findings, this compound has demonstrated genotoxic effects in vivo. It produced positive results in the in vivo mouse micronucleus test, indicating it is clastogenic. fda.govnih.govfda.gov Clastogenicity refers to the induction of structural chromosomal aberrations. who.int Studies have shown that this compound, like other NRTIs, can induce chromosomal aberrations in vivo. researchgate.net
Carcinogenicity in Animal Models (e.g., liver tumors, urinary bladder tumors)
Carcinogenicity studies in animal models have investigated the potential of this compound to cause tumors. In 2-year carcinogenicity studies in mice and rats, this compound was not found to be carcinogenic at doses producing exposures significantly higher than human exposure at the recommended clinical dose. fda.gov However, benign and malignant liver tumors in mice and rats, as well as malignant urinary bladder tumors in male rats, were observed at much higher exposure levels. fda.gov Carcinogenesis can occur through various mechanisms, including DNA damage and chronic inflammation. mdpi.comoaepublish.com Animal models, particularly rodents, are commonly used to study chemical carcinogenesis, including bladder cancer induced by various compounds. oaepublish.comnih.gov
Genomic Instability and Mutation Rates
The incorporation of NRTIs into nuclear DNA is proposed to result in genotoxic and mutagenic effects, including the induction of genomic instability and increased mutation rates. researchgate.net Compromised integrity of both nuclear and mitochondrial DNA is linked to genomic instability. researchgate.net While some studies focus on mutation rates in pathogens like Mycobacterium tuberculosis or the impact of viral proteins on mutation rates oup.comunipd.it, the mechanism by which this compound directly influences host genomic instability and mutation rates involves its incorporation into host DNA due to its structural similarity to natural nucleosides. fda.govresearchgate.net
Other Noted Toxicities (Mechanistic Inquiries)
Beyond genotoxicity and carcinogenicity, this compound is associated with other significant toxicities, often linked to mitochondrial dysfunction. nih.govwww.gov.uk
Myopathy
Myopathy, or muscle weakness and pain, is a known adverse effect associated with this compound use, similar to other NRTIs like zidovudine. mdpi.com The mechanism underlying this compound-induced myopathy is strongly linked to mitochondrial toxicity. nih.govwww.gov.uk this compound triphosphate inhibits cellular DNA polymerases, including DNA polymerase gamma (DNA pol γ), which is responsible for the replication of mitochondrial DNA (mtDNA). nih.govfda.gov Inhibition of DNA pol γ leads to depletion or dysfunction of mitochondria, impairing their ability to carry out essential functions like energy production and fatty acid metabolism. nih.govpharmgkb.org This mitochondrial failure in muscle tissue contributes to the development of myopathy. nih.gov Studies on zidovudine, which shares a similar mechanism of mitochondrial toxicity, have shown damage to mitochondria through both short-term effects on the respiratory chain and long-term alterations of mtDNA. nih.gov
Table: Summary of this compound Genotoxicity Findings
| Assay Type | Test System | Metabolic Activation | Result | Key Finding |
| In Vitro Gene Mutation | Ames, E. coli, CHO/HGPRT | +/- | Negative | Not mutagenic in these specific assays. |
| In Vitro Clastogenesis | Human lymphocytes | - | Positive | Elevated frequency of chromosome aberrations. fda.govnih.govfda.gov |
| In Vitro Cell Transformation | Mouse fibroblasts | +/- | Positive | Increased frequency of transformed foci. fda.govnih.govfda.gov |
| In Vivo Genotoxicity | Mouse micronucleus test | Not specified | Positive | Clastogenic effect observed. fda.govnih.govfda.gov |
| In Vivo Chromosomal Aberrations | Rat bone marrow cells | Not specified | Negative | No effect observed at tested dose. oup.com |
Table: Summary of this compound Carcinogenicity Findings in Animal Models
| Species | Tumor Type | Exposure Level (relative to human clinical dose) | Finding |
| Mice | Benign and malignant liver tumors | 250 times | Observed at this high exposure. fda.gov |
| Rats | Benign and malignant liver tumors | 732 times | Observed at this high exposure. fda.gov |
| Male Rats | Malignant urinary bladder tumors | 732 times | Observed at this high exposure. fda.gov |
| Mice, Rats | General Carcinogenicity | 39 and 168 times, respectively | Noncarcinogenic at these exposure levels. fda.gov |
Immune Reconstitution Inflammatory Syndrome (IRIS) Mechanisms
Immune Reconstitution Inflammatory Syndrome (IRIS) is a paradoxical clinical worsening that occurs in some HIV-infected patients after initiating effective antiretroviral therapy, including regimens containing this compound. wikipedia.orgsketchfab.commims.comguidetoimmunopharmacology.orgnih.gov This phenomenon is attributed to the recovery of the immune system, particularly the increase in CD4+ T cell counts, which mounts an inflammatory response to pre-existing opportunistic infections or antigens that were present before the initiation of HAART. wikipedia.orgmims.comguidetoimmunopharmacology.orgnih.govmims.com
The exact mechanisms driving IRIS are not yet fully understood, but several hypotheses exist. One central concept is that the restored immune system, previously suppressed by HIV, begins to recognize and react vigorously to pathogens or microbial antigens that were present at subclinical levels or were poorly contained during the period of immunodeficiency. wikipedia.orgnih.govmims.com This leads to an exaggerated inflammatory response at the site of the infection or antigen presence.
Key factors contributing to the development of IRIS include a low baseline CD4+ T cell count and a high viral load before starting HAART, as well as a rapid increase in CD4+ T cell counts and a significant drop in viral load after therapy initiation. wikipedia.org The rapid proliferation and activation of CD4+ T cells, particularly memory cells, upon viral suppression are thought to play a significant role. wikipedia.orgguidetoimmunopharmacology.org
While IRIS can be triggered by various opportunistic pathogens, including mycobacteria (such as Mycobacterium tuberculosis and Mycobacterium avium complex), viruses (like cytomegalovirus and varicella zoster virus), fungi (Cryptococcus neoformans), and protozoa, the underlying mechanism involves a dysregulation of the immune response. nih.gov This dysregulation may involve an imbalance between pro-inflammatory and anti-inflammatory cytokines. wikipedia.org Some research suggests that hyper-responsiveness of the innate immune system to T cell help might also contribute to the inflammatory cascade observed in IRIS. mims.com
This compound, as a component of HAART that effectively reduces viral load and allows for immune recovery, can therefore be associated with the development of IRIS in susceptible individuals, similar to other antiretroviral drugs that lead to robust immune reconstitution. wikipedia.orgsketchfab.commims.comguidetoimmunopharmacology.org The occurrence of IRIS highlights the complex interplay between viral suppression, immune recovery, and the host's response to residual or unmasked pathogens.
Bone Marrow Suppression (less common than zidovudine)
Bone marrow suppression, characterized by a decrease in the production of blood cells, is a known adverse effect of some NRTIs. While this compound can cause hematological abnormalities, it is generally considered less myelosuppressive compared to zidovudine. uni.lumims.com
The mechanism of bone marrow suppression by NRTIs like this compound and zidovudine is linked to their interference with cellular DNA polymerases, particularly mitochondrial DNA polymerase gamma (DNA pol γ). mims.comwikipedia.orgwikidata.orguni.lu DNA pol γ is essential for the replication of mitochondrial DNA (mtDNA). Inhibition of this enzyme can lead to mtDNA depletion and mitochondrial dysfunction in various tissues, including bone marrow progenitor cells. mims.comwikipedia.orgwikidata.org
However, the differential myelosuppression between this compound and zidovudine is not solely explained by mitochondrial toxicity, as both drugs can affect mitochondrial function. A key distinction lies in their impact on nuclear DNA synthesis in bone marrow cells. Studies have shown that this compound is incorporated into nuclear DNA of bone marrow cells at significantly lower levels (50- to 100-fold less) compared to zidovudine when cells are exposed to similar drug concentrations. mims.com This lower incorporation into nuclear DNA in hematopoietic precursor cells is a proposed mechanism for the reduced myelotoxicity of this compound relative to zidovudine. mims.com
Zidovudine's myelosuppressive effects, particularly anemia and neutropenia, are thought to involve the suppression of erythropoiesis and inhibition of erythroid stem cells, in addition to mitochondrial toxicity. nih.govnih.gov While this compound can also cause mitochondrial toxicity leading to other adverse effects like peripheral neuropathy and lactic acidosis, its lesser impact on bone marrow appears to be related to this reduced interference with nuclear DNA synthesis in these rapidly dividing cells compared to zidovudine. mims.commims.comwikipedia.orgwikidata.orguni.lu
The relative myelosuppression of this compound compared to zidovudine is a significant clinical difference that influenced treatment decisions, particularly in patients with pre-existing hematological issues or those receiving concomitant medications that could exacerbate myelosuppression. wikipedia.org
Development of Stavudine Analogues and Derivatives
Structure-Activity Relationship Studies for Enhanced Antiviral Efficacy
Structure-Activity Relationship (SAR) studies on stavudine analogues aim to identify the chemical modifications that influence their antiviral potency. These studies involve synthesizing compounds with variations at different positions of the this compound structure and evaluating their inhibitory activity against HIV.
Research has explored modifications at various positions of the thymidine base and the sugar moiety. For instance, studies on 4'-substituted 2',3'-didehydro-3'-deoxythymidine (4'-substituted D4T) analogs have revealed that the nature of the substituent at the 4' position significantly impacts antiviral efficacy. nih.govasm.org Among a series of 4'-substituted D4T analogs, the 4'-ethynyl substituted compound demonstrated enhanced antiviral effects compared to the parental this compound. nih.govnih.gov Other substitutions at the 4' position, such as methyl, vinyl, methylethynyl, chloroethynyl, allyl, and cyano groups, showed varying degrees of activity, with some being less active or inactive compared to this compound. nih.gov
Quantitative SAR (QSAR) studies on related viral reverse transcriptase inhibitors, such as TSAO derivatives, have indicated that antiviral activities can be significantly correlated with the hydrophobic and electronic properties of the molecules. tandfonline.com While this study focused on a different class of inhibitors, it highlights the general principle that physicochemical properties play a crucial role in the antiviral activity of nucleoside analogs.
Phosphoramidate derivatives of this compound have also been synthesized as potential prodrugs designed to improve intracellular delivery of the active monophosphate. nih.govresearchgate.net Some phenyl phosphoramidate derivatives of this compound have shown potent anti-HIV activity at nanomolar concentrations. nih.gov Myristoylated derivatives of this compound have also been explored, with some bi-functional prodrugs exhibiting potent anti-HIV-1 activity. researchgate.net
Strategies for Reduced Cytotoxicity and Mitochondrial Toxicity
A major limitation of this compound is its association with mitochondrial toxicity, which can lead to severe adverse effects like peripheral neuropathy and lactic acidosis. nih.govnih.govnatap.orgacs.org Strategies for reducing cytotoxicity and mitochondrial toxicity in this compound analogues focus on designing compounds that are less prone to interfering with host cellular DNA polymerases, particularly mitochondrial DNA polymerase gamma (Pol γ), while retaining potent inhibition of HIV reverse transcriptase. acs.orgasm.org
Studies have shown that the incorporation of nucleoside analogues by mitochondrial DNA polymerase is strongly correlated with their toxic side effects. acs.org The relative potency of inhibition of mitochondrial DNA synthesis varies among different nucleoside reverse transcriptase inhibitors (NRTIs), with this compound showing a higher potential for inhibition compared to some other NRTIs like lamivudine and tenofovir. acs.orgasm.org
Modifications to the this compound structure, such as the introduction of a 4'-ethynyl group, have shown promise in reducing cellular and mitochondrial toxicity compared to the parental compound. nih.govnih.govasm.org This suggests that specific structural alterations can influence the interaction of the this compound analogue triphosphate with host polymerases, thereby reducing off-target toxicity.
Further modifications of modified nucleosides have been observed to reduce toxicity and, in some cases, enhance antiviral activity. acs.org This indicates that a balance between antiviral efficacy and host cell toxicity can potentially be achieved through careful structural design.
Evaluation of Novel 4'-Substituted this compound Analogs (e.g., 4'-ethynyl D4T)
Novel 4'-substituted this compound analogs have been a key area of research to find compounds with improved therapeutic profiles. The evaluation of these analogs involves assessing their antiviral potency against various HIV strains, their cytotoxicity in different cell lines, and their metabolic fate.
Among the 4'-substituted D4T analogs, 4'-ethynyl D4T (also known as 4'-Ed4T, festinavir, or censavudine) has been extensively studied. nih.govnih.govasm.orgasm.orgasm.org Research indicates that 4'-ethynyl D4T is significantly more potent against HIV-1 replication in cell culture compared to this compound, with studies reporting a fivefold to tenfold increase in antiviral effect. nih.govnih.govasm.orgnatap.org
Furthermore, 4'-ethynyl D4T has demonstrated reduced cellular and mitochondrial toxicity compared to this compound. nih.govnih.govasm.org This reduced toxicity is potentially linked to its triphosphate metabolite having a weaker inhibitory effect on major host DNA polymerases. asm.org
The mechanism of action of 4'-ethynyl D4T involves phosphorylation by cellular kinases, including thymidine kinase 1 (TK-1), to its active triphosphate form. nih.govnih.gov Studies have shown that TK-1 phosphorylates 4'-ethynyl D4T more efficiently than this compound. nih.govnih.gov The triphosphate of 4'-ethynyl D4T inhibits HIV-1 reverse transcriptase more efficiently than this compound triphosphate. asm.org Kinetic studies suggest that 4'-ethynyl D4T triphosphate has a higher binding affinity for HIV-1 reverse transcriptase. asm.org Structural modeling indicates that the 4'-ethynyl group may fit into a hydrophobic pocket in the reverse transcriptase active site, contributing to enhanced binding and potentially affecting translocation. acs.orgasm.org
Evaluation of 4'-ethynyl D4T has also included assessing its activity against drug-resistant HIV-1 strains. asm.orgnatap.org It has shown activity against certain resistant variants, including those with the Q151M complex mutation, which confers resistance to several other NRTIs. natap.org
Other 4'-substituted thiostavudines, including 4'-cyano and 4'-ethynyl analogues, have also been synthesized and evaluated for anti-HIV activity. nih.gov While the activity of the 4'-ethynyl thiothis compound was comparable to this compound in one study, it was not as active as 4'-ethynylthis compound. nih.gov
The synthesis and evaluation of carbocyclic this compound analogues with a geminal-difluoromethylidene group at the 5' position and an ethynyl group at the 4' position have also been explored, demonstrating the ongoing efforts to create novel structures with potential antiviral activity. oup.com
Antiviral Activity and Cytotoxicity of this compound and 4'-Ethynyl D4T
| Compound | EC₅₀ (µM) (MT-4 cells) | CC₅₀ (µM) (MT-4 cells) | Selectivity Index (CC₅₀/EC₅₀) | Source |
| This compound (d4T) | 0.31 | 79 | 255 | natap.org |
| 4'-Ethynyl D4T | 0.070 | >100 | >1428 | natap.org |
Note: EC₅₀ represents the 50% effective concentration, and CC₅₀ represents the 50% cytotoxic concentration. Selectivity Index is a measure of the compound's antiviral activity relative to its cytotoxicity.
This table illustrates that 4'-ethynyl D4T exhibits a lower EC₅₀, indicating higher antiviral potency, and a higher CC₅₀ (or > value), indicating lower cytotoxicity, compared to this compound in MT-4 cells. This results in a significantly higher selectivity index for 4'-ethynyl D4T, suggesting a better therapeutic window.
Research Methodologies and Experimental Models
In Vitro Cell Culture Models for Antiviral Activity and Toxicity Assessment
In vitro cell culture models have been extensively used to assess the antiviral activity and cytotoxicity of stavudine. These models provide a controlled environment to study the direct effects of the compound on HIV-1 replication and host cells.
Studies have measured the antiviral activity of this compound in various cell types, including peripheral blood mononuclear cells, monocytic cells, and lymphoblastoid cell lines. The concentration required to inhibit HIV-1 replication by 50% (EC50) has been reported to range from 0.0009 to 4 µM against laboratory and clinical isolates of HIV-1. fda.govfda.gov
The cytotoxicity of this compound has also been evaluated in cell cultures. For instance, studies in mock-infected MT-4 cells and PBMCs showed that this compound was more cytotoxic than some related compounds, with 50% cytotoxic concentrations (CC50) of 79 µM in MT-4 cells and 28 µM in PBMCs. asm.org In HepG2 liver cells and normal skeletal muscle cells, this compound demonstrated lower CC50 values compared to tenofovir, indicating higher cytotoxicity in these cell types. nih.gov Evaluation of hematopoietic toxicity revealed that this compound was more cytotoxic towards erythroid progenitor cells than tenofovir. nih.gov The inhibitory activity against myeloid cell lineage, in order of decreasing severity among tested NRTIs, was consistently ddC > ZDV > d4T > tenofovir > 3TC. nih.gov
Combinations of this compound with other antiretroviral agents have also been investigated in vitro. This compound in combination with abacavir, didanosine, tenofovir, or zalcitabine has shown additive to synergistic anti-HIV-1 activity in cell culture. fda.govfda.gov Conversely, ribavirin, at concentrations of 9-45 µM, has been shown to reduce the anti-HIV-1 activity of this compound by 2.5- to 5-fold in vitro. fda.govfda.gov
Data on in vitro antiviral activity and cytotoxicity:
| Cell Type | HIV-1 Strain/Isolate | EC50 (µM) | CC50 (µM) | Reference |
| Peripheral Blood Mononuclear Cells | Laboratory/Clinical | 0.0009 - 4 | - | fda.govfda.gov |
| Monocytic Cells | Laboratory/Clinical | 0.0009 - 4 | - | fda.govfda.gov |
| Lymphoblastoid Cell Lines (e.g., MT-4) | Laboratory/Clinical | 0.0009 - 4 | 79 | fda.govfda.govasm.org |
| PBMCs | IIIB, Ba-L | 0.0019, 0.0076 | 28 | asm.org |
| HepG2 cells | - | - | <398 | nih.gov |
| Normal skeletal muscle cells | - | - | <870 | nih.gov |
| Erythroid progenitor cells | - | - | 0.06-5 | nih.gov |
Note: EC50 range is for antiviral activity, CC50 values indicate cytotoxicity.
Animal Models in Preclinical Safety and Efficacy Evaluation (e.g., mice, rats for genotoxicity and carcinogenicity)
Animal models, particularly mice and rats, have been instrumental in the preclinical evaluation of this compound's safety and efficacy, including assessments of genotoxicity and carcinogenicity. fda.govhiv.goveuropa.euca.goveupati.eugwern.net
In 2-year carcinogenicity studies conducted in mice and rats, this compound was found to be noncarcinogenic at doses yielding exposures (AUC) approximately 39 and 168 times, respectively, the human exposure at the recommended clinical dose. fda.govhiv.goveuropa.eu However, at higher exposure levels (250 times in mice and 732 times in rats compared to human therapeutic exposure), benign and malignant liver tumors were observed in both species. fda.govhiv.goveuropa.eu Additionally, malignant urinary bladder tumors occurred in male rats at these elevated exposure levels. fda.govhiv.goveuropa.eu
Studies have also investigated the genotoxic potential of this compound in animal models. This compound was found to be clastogenic in an in vivo micronucleus assay in bone marrow cells of mice following oral administration at dosages of 600 to 2000 mg/kg/day for 3 days. fda.gov
Reproduction studies in rats and rabbits with exposures significantly exceeding clinical levels (up to 399 and 183 times, respectively, based on Cmax) revealed no evidence of teratogenicity. fda.govhiv.gov However, an increased incidence of a common skeletal variation (unossified or incomplete ossification of sternebra) was noted in rat fetuses at 399 times human exposure, but not at 216 times. fda.govhiv.gov A slight post-implantation loss was also observed in rats at 216 times human exposure, with no such effect at approximately 135 times. fda.govhiv.gov An increase in early neonatal mortality (birth to day 4) in rats occurred at 399 times human exposure, while survival was unaffected at approximately 135 times. hiv.gov
Animal models have also been used to study the toxicity profile of this compound and its derivatives. A study evaluating a this compound derivative, stampidine (STAMP), in BALB/c and CD-1 mice found it to be well-tolerated with no detectable acute or subacute toxicity at high single or daily doses over several weeks. asm.org
Mechanistic Studies using Pre-Steady-State Kinetics (e.g., DNA Polymerase Gamma activity)
Pre-steady-state kinetics is a powerful technique used to investigate the rapid steps of enzyme reactions, providing detailed insights into the mechanism of action and inhibition. This approach has been applied to study the interaction of this compound triphosphate (d4TTP), the active metabolite of this compound, with DNA polymerase gamma (Pol γ), a key enzyme in mitochondrial DNA replication. nih.govnih.govoup.comasm.orgacs.org
Studies utilizing pre-steady-state kinetics have focused on understanding how d4TTP competes with the natural substrate, deoxythymidine triphosphate (dTTP), and how mutations in Pol γ can affect this interaction and contribute to toxicity. nih.govnih.govasm.org For example, research on the R964C mutation of human DNA polymerase γ, which has been linked to this compound-mediated mitochondrial toxicity, employed pre-steady-state kinetics to assess the incorporation of both dTTP and d4TTP. nih.govnih.govasm.org
The R964C polymerase γ holoenzyme demonstrated a 33% decrease in dTTP incorporation efficiency compared to the wild-type enzyme. nih.govnih.gov Crucially, this mutant also showed a threefold lower discrimination against d4TTP relative to the wild-type Pol γ. nih.govnih.gov This reduced ability of the mutant enzyme to distinguish between the natural nucleotide and the this compound metabolite provides a mechanistic explanation for the increased susceptibility to mitochondrial toxicity observed in individuals with this mutation. nih.govnih.govasm.org
Comparison of Wild-Type and R964C Mutant Pol γ Holoenzyme Kinetics (Pre-Steady-State):
| Enzyme Type | Substrate | Incorporation Efficiency (Relative to Wild-Type) | Discrimination Against d4TTP (Relative to Wild-Type) | Reference |
| Wild-Type Pol γ | dTTP | 100% | 100% | nih.govnih.gov |
| R964C Mutant Pol γ | dTTP | 67% | - | nih.govnih.gov |
| Wild-Type Pol γ | d4TTP | - | 100% | nih.govnih.gov |
| R964C Mutant Pol γ | d4TTP | - | 33% (threefold lower discrimination) | nih.govnih.gov |
Note: Data derived from pre-steady-state kinetic analyses.
Other pre-steady-state kinetic studies have examined the interaction of d4TTP with HIV-1 reverse transcriptase, showing that a this compound analogue, 4′-ethynyl-d4T triphosphate (4′-Ed4TTP), had lower Kd values than d4TTP with both wild-type and M184V mutant RTs. asm.org This suggests stronger binding of the analogue to the enzyme. asm.org
Human Placenta Models for Drug Transfer Studies
Understanding the transfer of drugs across the placenta is critical, particularly for medications administered during pregnancy. Human placenta models, including ex vivo perfusion systems, have been used to study the placental transfer of this compound. europa.eueuropa.eunih.govmdpi.comsemanticscholar.orgroyalsocietypublishing.org
An ex vivo study utilizing a term human placenta model demonstrated that this compound reaches the fetal circulation primarily through simple diffusion. europa.eueuropa.eu This indicates that passive movement across the placental barrier is a significant factor in this compound transfer from the maternal to the fetal compartment.
Animal studies have corroborated placental transfer. A study in rats showed that this compound is transferred to the fetus through the placenta, with the concentration in fetal tissue being approximately half the concentration in maternal plasma. hiv.goveuropa.eueuropa.eu In primates (pig-tailed macaques), the ratio of fetal plasma concentrations to maternal plasma concentrations was approximately 0.80. hiv.gov
These models provide valuable data on the potential fetal exposure to this compound when administered to pregnant individuals. The ex vivo human placental perfusion model, while not providing pharmacokinetic data, offers insights into drug transfer within the physiological tissue architecture of the human placenta. nih.govsemanticscholar.org
Evolving Research Perspectives and Future Trajectories
Research into Minimizing Long-Term Toxicity with Reduced Dosing Strategies (e.g., low-dose stavudine)
Long-term use of this compound has been associated with significant toxicities, including peripheral neuropathy and lipoatrophy, linked to mitochondrial toxicity. Research has investigated the potential of reduced dosing strategies to mitigate these adverse effects while maintaining antiviral efficacy.
Studies have shown that lowering the dose of this compound from 40 mg to 30 mg twice daily can lead to a significant increase in mitochondrial DNA content in peripheral blood mononuclear cells and adipocytes while preserving virologic suppression. nih.gov This led the World Health Organization (WHO) to recommend a reduction in the adult dose to 30 mg twice daily, irrespective of weight, in 2007. nih.govnih.govasm.org
Further research has explored even lower doses, such as 20 mg twice daily. Some studies suggest that this lower dose might offer comparable viral suppression with a reduced risk of toxicity and side effects, at a lower cost than alternatives like tenofovir. researchgate.net A meta-analysis in 2007 indicated that a lower this compound dose of 20 mg for adults weighing less than 60 kg and 30 mg for those over 60 kg twice daily resulted in a markedly lower risk of lipoatrophy while maintaining antiviral efficacy. nih.govasm.org
Clinical trials have been proposed and conducted to compare low-dose this compound regimens with newer agents like tenofovir in terms of viral suppression and toxicity. researchgate.net For instance, a study in South Africa compared the incidence and severity of peripheral neuropathy between patients receiving 40 mg and 30 mg doses of this compound. The incidence of peripheral neuropathy was significantly lower in the 30 mg group compared to the 40 mg cohort. nih.gov
While studies suggest that reducing the dose to 30 mg has shown a lower incidence of some adverse events and a lower risk of discontinuation within the first year of ART, further evidence is needed from resource-limited settings to determine the benefit of a routine 20 mg dose, particularly for patients with low body weights. nih.gov
Data from a study in Malawi with universal 30 mg dosing still showed that this compound-associated toxicities were common, indicating that while dose reduction helps, toxicity remains a concern. plos.org
Research into reduced dosing aims to find an optimal balance between efficacy, reduced mitochondrial toxicity, and cost-effectiveness, particularly in settings where access to more expensive alternatives is limited.
Comparative Effectiveness and Safety Research in Resource-Limited Settings
In resource-limited settings, decisions regarding first-line antiretroviral therapy regimens often involve balancing cost, the need for laboratory monitoring, and the frequency and severity of side effects against effectiveness. plos.org this compound was initially widely used in these settings due to its lower cost and minimal requirements for laboratory monitoring compared to zidovudine. plos.org
Despite its known toxicities, this compound has remained a cornerstone of treatment in some resource-limited settings due to the lack of affordable alternatives and its availability in generic fixed-dose combinations. tandfonline.comcapes.gov.br Studies in these regions have shown virological success with this compound-based highly active antiretroviral therapy. tandfonline.comcapes.gov.br For example, observational studies from Mozambique, Cameroon, and Malawi have reported low rates of treatment failure and switching to second-line regimens with this compound-based regimens. plos.org
However, comparative studies with newer agents like tenofovir have highlighted the limitations of this compound. A cohort study in South Africa comparing tenofovir, this compound (30 mg dose), and zidovudine found that tenofovir appeared to perform better, particularly with less drug substitution and mortality than either this compound or zidovudine in routine care. plos.org The adjusted hazard ratio for a single drug substitution was significantly higher for this compound and zidovudine compared to tenofovir after the initial 6 months of ART. plos.org Similarly, the adjusted hazard ratio for mortality was higher for this compound compared to tenofovir. plos.org
Despite these findings, the transition from this compound to tenofovir in resource-limited settings has been slow, largely due to the higher cost of tenofovir and challenges in managing its potential toxicities, such as renal insufficiency. nih.gov
Investigation of Biomarkers for Early Detection of this compound-Related Toxicities
Given the significant long-term toxicities associated with this compound, research is ongoing to identify biomarkers that can facilitate the early detection of these adverse effects, potentially allowing for timely intervention or switching to alternative regimens before severe complications develop.
Mitochondrial toxicity is a key mechanism underlying many this compound-associated side effects, including peripheral neuropathy and lipodystrophy. drugs.comoup.com Depletion of mitochondria has been linked to mitochondrial dysfunction. nih.gov Studies have investigated whether mitochondrial DNA (mtDNA) levels in peripheral blood can serve as a biomarker for this compound-associated mitochondrial toxicities. nih.gov
A study in Malawi found that healthy controls had a higher median mtDNA/nuclear DNA (nDNA) ratio compared to HIV/AIDS patients on this compound-containing ART, particularly those presenting with peripheral neuropathy or high lactate levels. nih.gov Significant differences in median mtDNA/nDNA ratios were observed between patients with high and normal lactate levels. nih.gov These findings suggest that peripheral blood mtDNA/nDNA ratio could be a marker of mitochondrial toxicities associated with this compound. nih.gov
Further research into biomarkers could potentially help identify individuals at higher risk of developing this compound-related toxicities, allowing for personalized treatment approaches and closer monitoring. This could be particularly valuable in resource-limited settings where frequent clinical monitoring and access to advanced diagnostic tools may be limited.
Ethical Considerations in this compound Research and Use in Global Health
The continued use of this compound in resource-limited settings, despite its known long-term toxicities and the availability of less toxic alternatives in wealthier nations, raises important ethical considerations in global health. philarchive.orgnih.gov
One key ethical debate revolves around the principle of justice in global health: is it ethically preferable to provide a larger number of people with cheaper, potentially more toxic treatments (like this compound) or to restrict treatment to a smaller group to provide more expensive but safer alternatives? philarchive.org This issue was highlighted in controversies surrounding clinical trials in developing countries, where questions were raised about the standard of care provided to control groups when effective treatments were available elsewhere. nih.govnih.gov
The use of this compound in resource-limited settings can be seen as an example of a "double standard" in healthcare, where patients in lower-income countries receive regimens that are no longer recommended in high-income countries due to toxicity concerns. msfaccess.orgnih.gov While the affordability of this compound has been crucial for expanding access to ART in these regions, the long-term health consequences of its toxicities are a significant concern. plos.orgmsfaccess.orgplos.org
Ethical considerations in this compound research and use include:
Informed Consent: Ensuring that patients in resource-limited settings are fully informed about the potential risks and benefits of this compound, as well as the availability (or lack thereof) of alternative treatments, is crucial for obtaining truly informed consent. drugsandalcohol.ie
Access and Equity: The disparity in access to newer, less toxic drugs between resource-rich and resource-limited settings raises questions of equity in global health. philarchive.orgnih.govoup.com
Research Ethics: When conducting research involving this compound in resource-limited settings, researchers must adhere to the highest ethical standards, ensuring that participants are not exploited and that the research is relevant to the health needs of the community. nih.govdrugsandalcohol.ieresearchgate.net This includes careful consideration of control groups in clinical trials and ensuring that participants have access to the best available treatment both during and after the study. nih.gov
Sustainability: The long-term costs associated with managing this compound-related toxicities can impact the sustainability of ART programs in resource-limited settings. msfaccess.org
Addressing these ethical challenges requires ongoing dialogue among researchers, policymakers, healthcare providers, and affected communities to ensure that decisions regarding the use of this compound and the introduction of newer drugs prioritize patient well-being and health equity. philarchive.orgdrugsandalcohol.ieoup.com
Q & A
Basic: How should researchers design experiments to assess Stavudine’s stability under suboptimal storage conditions?
Answer:
To evaluate this compound degradation, replicate real-world storage scenarios (e.g., high humidity, temperatures >25°C) using HPLC for quantitative analysis of active pharmaceutical ingredient (API) levels . Experimental variables should include:
- Packaging types (e.g., non-manufacturer containers, anonymized packaging to mimic patient behavior).
- Environmental stressors (e.g., 40°C/75% relative humidity for subtropical regions).
- Sampling intervals (e.g., biweekly analysis over 12 weeks).
Data should be normalized to baseline API concentrations, and degradation products (e.g., thymine) quantified. Include a control group with manufacturer-recommended storage conditions.
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


