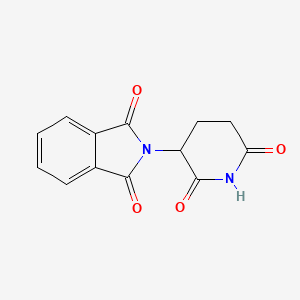
Thalidomide
Description
Structural and Functional Relationships
This compound’s biological activity is intricately tied to its stereochemistry. The (S)-enantiomer binds preferentially to cereblon, initiating ubiquitination of transcription factors like SALL4 and IKZF1/3, which regulate limb development and immune responses. In contrast, the (R)-enantiomer exhibits weaker binding affinity, contributing primarily to sedative effects. Despite in vivo racemization—a process where enantiomers interconvert—the (S)-form dominates in therapeutic contexts due to its higher bioavailability.
Properties
IUPAC Name |
2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UEJJHQNACJXSKW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(=O)NC(=O)C1N2C(=O)C3=CC=CC=C3C2=O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H10N2O4 | |
| Record name | THALIDOMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/21096 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9022524 | |
| Record name | Thalidomide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022524 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
258.23 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Thalidomide appears as needles or white powder. (NTP, 1992), Solid | |
| Record name | THALIDOMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/21096 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Thalidomide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015175 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
less than 1 mg/mL at 72 °F (NTP, 1992), In water, approximately 2X10-4 mol/L; 45-60 mg/L at 25 °C, Sparingly soluble in methanol, ethanol, acetone, ethyl acetate, butyl acetate, glacial acetic acid. Very soluble in DMF. Practically insoluble in ether, chloroform, benzene., Very soluble in dioxane, pyridine, 2.55e+00 g/L | |
| Record name | THALIDOMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/21096 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Thalidomide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01041 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | THALIDOMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3586 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thalidomide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015175 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Needles | |
CAS No. |
50-35-1, 841-67-8, 2614-06-4 | |
| Record name | THALIDOMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/21096 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Thalidomide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-35-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Thalidomide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050351 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Thalidomide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01041 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | thalidomide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758479 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | thalidomide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=527179 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | l-Thalidomide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=91730 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | D-Thalidomide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=91729 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | thalidomide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=66847 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Thalidomide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022524 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Thalidomide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.029 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | THALIDOMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4Z8R6ORS6L | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | THALIDOMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3586 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thalidomide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015175 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
516 to 520 °F (NTP, 1992), 270 °C | |
| Record name | THALIDOMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/21096 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Thalidomide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01041 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | THALIDOMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3586 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Thalidomide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015175 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action
Target of Action
Thalidomide primarily targets a protein called Cereblon (CRBN) . CRBN is a substrate receptor of the Cullin-RING E3 Ubiquitin ligase complex. It plays a crucial role in various biological processes, including limb outgrowth, teratogenicity, and tumorigenesis.
Mode of Action
This compound binds to CRBN, inhibiting the ligase’s normal activity by preventing endogenous substrates from binding, thereby protecting them from degradation. It also recruits transcription factors such asIKZF1 and IKZF3 to the enzyme’s active site, promoting their degradation. This interaction leads to the modulation of various downstream processes, including the degradation of an unexpectedly wide range of transcription factors, such as SALL4 .
Biochemical Pathways
This compound affects several biochemical pathways. It has been found to promote the degradation of transcription factors, leading to the complete removal of SALL4 from cells. This action impacts the pathways involved in limb development. Additionally, this compound is effective in T helper cell (Th) immunoregulation by preferentially inducing Th2 cytokine production and inhibiting Th1 cytokine production in peripheral mononuclear cells.
Pharmacokinetics
This compound exhibits absorption rate-limited pharmacokinetics or the “flip-flop” phenomenon. Following a single dose of 200 mg in healthy male subjects, the peak concentration (Cmax) and the area under the curve (AUC∞) were calculated to be 2.00 ± 0.55 mg/L and 19.80 ± 3.61 mg*h/mL respectively. This compound is minimally metabolised by the liver, but is spontaneously hydrolysed into numerous renally excreted products.
Result of Action
The molecular and cellular effects of this compound’s action are diverse. It displays immunosuppressive and anti-angiogenic activity through modulating the release of inflammatory mediators like tumor necrosis factor-alpha (TNF-a) and other cytokine action. This compound is also known for its severe teratogenic effects, causing skeletal deformities such as amelia (absence of legs and/or arms), absence of bones, and phocomelia (malformation of the limbs).
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, metabolic breakdown by CYP450 in the liver is required for this compound to be active. Furthermore, in rural areas of the world that lack extensive medical surveillance initiatives, this compound treatment of pregnant women with leprosy has continued to cause malformations.
Biochemical Analysis
Biochemical Properties
Thalidomide exists in two mirror-image forms: a racemic mixture of ®- and (S)-enantiomers. The ®-enantiomer has sedative effects, whereas the (S)-isomer is teratogenic. Under biological conditions, these isomers interconvert, rendering the separation of the isomers before use ineffective. This compound interacts with a protein called cereblon (CRBN), a component of an E3 ubiquitin ligase complex. The binding of this compound to CRBN affects the ubiquitin ligase activity of the complex.
Cellular Effects
This compound has been shown to have various effects on cells. It can inhibit the production of TNF-α and replication of HIV. It also has anti-angiogenic activity, suggesting that this compound can inhibit the growth of new blood vessels. This compound’s binding to CRBN can lead to the degradation of certain proteins, affecting cell survival and proliferation.
Molecular Mechanism
This compound initiates its effects by binding to CRBN, inhibiting its ubiquitin ligase activity. This binding alters the substrate specificity of the E3 ubiquitin ligase complex, leading to the degradation of specific proteins. For example, this compound and its derivatives can induce the degradation of transcription factors like Ikaros and Aiolos, which are crucial for the development of lymphoid cells and the survival of multiple myeloma cells.
Temporal Effects in Laboratory Settings
In laboratory settings, this compound has shown temporal effects. For instance, in patients with transfusion-dependent β-thalassemia, hemoglobin concentrations achieved a median elevation after 12 weeks of this compound treatment. This suggests that this compound’s effects can change over time in a laboratory setting.
Metabolic Pathways
This compound undergoes biotransformation by non-enzymatic hydrolysis and enzyme-mediated hydroxylation to form a multitude of metabolites
Biological Activity
Thalidomide, originally marketed in the late 1950s as a sedative and treatment for morning sickness, is now recognized for its complex biological activity, which includes immunomodulation, anti-inflammatory effects, and anti-angiogenesis. Despite its notorious history of causing birth defects, recent research has revealed its potential therapeutic applications in various conditions, including multiple myeloma and leprosy. This article examines the biological activity of this compound, supported by case studies and detailed research findings.
This compound's biological effects are mediated through several mechanisms:
- Cytokine Modulation : this compound selectively inhibits the production of tumor necrosis factor-alpha (TNF-α) in human monocytes by promoting the degradation of TNF-α mRNA. This action is crucial in managing inflammatory conditions .
- Inhibition of NF-κB : this compound inhibits nuclear factor kappa B (NF-κB) activity by affecting I-κB kinase activity. NF-κB is pivotal in regulating immune responses and inflammatory cytokines such as IL-6 and IL-12 .
- T Cell Activation : The compound stimulates T cell proliferation, particularly enhancing CD8+ T cell responses while promoting a Th2 cytokine profile that favors IL-4 production over interferon-gamma (IFN-γ) .
- Anti-Angiogenic Properties : this compound has been shown to inhibit angiogenesis through its effects on vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), which may explain some of its teratogenic effects during fetal development .
Therapeutic Applications
This compound's unique properties have led to its reintroduction in clinical settings:
- Multiple Myeloma : this compound is utilized in treating relapsed-refractory multiple myeloma due to its ability to inhibit tumor growth and modulate the immune response .
- Erythema Nodosum Leprosum : It is effective in managing complications associated with leprosy, reducing inflammation and promoting healing .
- COVID-19 Treatment : Recent studies indicate that this compound may accelerate recovery from severe COVID-19 by reducing inflammation and modulating immune responses, particularly when combined with low-dose glucocorticoids .
Case Study 1: this compound Embryopathy
A study documented a woman who experienced this compound embryopathy after taking the drug during pregnancy. The findings highlighted the teratogenic effects linked to this compound's mechanism of action involving the degradation of transcription factors essential for limb development, such as SALL4 .
Case Study 2: COVID-19 Treatment Efficacy
In a clinical trial involving COVID-19 patients, this compound treatment resulted in a significant reduction in hospital stay duration and mechanical ventilation requirements compared to standard care. This was attributed to its anti-inflammatory effects .
Data Tables
| Mechanism of Action | Effect on Cytokines | Therapeutic Use |
|---|---|---|
| Inhibition of TNF-α | Reduces inflammation | Multiple Myeloma |
| Inhibition of NF-κB | Decreases IL-6 and IL-12 | Erythema Nodosum Leprosum |
| T Cell Activation | Enhances CD8+ T cell response | COVID-19 treatment |
| Anti-Angiogenic Properties | Inhibits VEGF and bFGF | Potential cancer therapies |
Scientific Research Applications
Clinical Applications
Thalidomide's therapeutic applications have expanded significantly since its initial ban. Below is a summary of its key uses:
Case Studies
- Angiogenesis Inhibition in AVMs : A prospective observational study on 18 patients with symptomatic AVMs showed that this compound reduced pain and bleeding significantly. After 19 months of treatment, one patient exhibited complete resolution of the AVM .
- Multiple Myeloma Treatment : A comprehensive analysis of this compound's role in treating multiple myeloma indicated improved patient outcomes when combined with other therapies like bortezomib (Velcade). This combination has been particularly beneficial for patients with advanced stages of the disease .
- Graft Versus Host Disease : In a randomized trial assessing this compound's efficacy as a prophylactic agent against chronic graft versus host disease, results indicated that while it was effective therapeutically, it paradoxically increased the incidence of GVHD when used preventively .
Comparison with Similar Compounds
Lenalidomide and Pomalidomide
- Structural Modifications: Lenalidomide features an amino group at the 4th position of the phthalimide ring, while pomalidomide adds a methoxy group .
- Mechanism: Both bind CRBN but exhibit enhanced specificity for degradation of Ikaros (IKZF1) and Aiolos (IKZF3), critical in multiple myeloma therapy.
- Efficacy : Lenalidomide shows a 60% response rate in relapsed myeloma, compared to thalidomide’s 32% .
- Safety : Lower neurotoxicity but higher risk of myelosuppression .
CC-885 and CC-90009
- Structural Modifications : CC-885 includes a urea and chloromethyl-phenyl group; CC-90009 further optimizes this scaffold .
- Mechanism : Degrade GSPT1, a translation termination factor, showing potent anti-leukemic activity (IC₅₀ < 10 nM in AML cell lines) .
- Advantage: No anti-angiogenic effects, avoiding this compound-associated thrombosis .
Apremilast
- Structural Modifications: Phthalimide replaced with a phthalazinone core .
- Mechanism : Targets phosphodiesterase-4 (PDE4) instead of CRBN, reducing TNF-α and IL-23 without teratogenic CRBN interactions .
Experimental Analogues
Quinazolinone-Based Derivatives (e.g., Avadomide)
Compound 5C (Anticonvulsant Analogue)
Compound 7a (Anticancer Agent)
- Structure : Thiourea-anilide hybrid .
- Efficacy : IC₅₀ of 10.32 µg/mL against MCF-7 cells, superior to this compound (11.26 µg/mL) .
Data Table: Key this compound Analogues
Research Findings and Clinical Implications
- Teratogenicity Avoidance : Analogues like lenalidomide and apremilast bypass SALL4 degradation, mitigating limb defects .
- Enhanced Specificity: CC-885’s GSPT1 targeting offers leukemia-selective cytotoxicity, sparing normal cells .
- Diverse Mechanisms : Apremilast’s PDE4 inhibition provides CRBN-independent anti-inflammatory effects, expanding therapeutic applications .
Preparation Methods
Original Synthesis and Early Modifications
Thalidomide [(±)-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione] was first synthesized in 1954 via a four-step process involving phthalic anhydride and L-glutamic acid. The initial method suffered from low yields (19%) due to intermediate purification requirements and the use of toxic reagents like sulfuryl chloride. Key steps included:
- Carbethoxylation : Reaction of phthalic anhydride with ethyl chloroformate to form N-carbethoxy-phthalimide.
- Condensation : Interaction with L-glutamic acid under basic conditions.
- Cyclization : Glutarimide ring closure using sodium amide in liquid ammonia.
This method’s inefficiency spurred efforts to eliminate the carbethoxylation step. By 2005, researchers achieved a 55% yield via a two-step pathway combining Boc-protected L-glutamic acid diester with Na/liquid NH3-mediated cyclization, followed by phthalic anhydride condensation.
Two-Step Facile Synthesis (2019)
A breakthrough in 2019 simplified the process to two steps with a 56% overall yield:
- Formation of N-phthaloyl-DL-glutamic acid (IV) :
- Cyclization to this compound :
Optimization Insights :
- Solvent selection (diphenyl ether vs. toluene) improved cyclization efficiency by 22%.
- Ammonium acetate proved superior to urea as an ammonia donor, reducing side-product formation.
Industrial-Scale Methodologies
One-Pot Synthesis (Patent WO2009083724A1)
This patent describes a one-pot method scalable to industrial production:
- Reaction : L-glutamine and phthalic anhydride in toluene with triethylamine.
- Dehydration : Acetyl chloride or acetic anhydride at 110–120°C.
- Yield : 55–75g per 100g glutamine (55–75%).
Advantages :
- Eliminates intermediate isolation.
- Uses cost-effective solvents (toluene) and reagents.
Microwave-Assisted Green Synthesis (2017)
A solvent-free, microwave-irradiated method achieved 68% yield in 30 minutes:
- Reagents : Phthalic anhydride, L-glutamic acid, ammonium chloride.
- Catalyst : 4-N,N-dimethylaminopyridine (DMAP).
- Conditions : 150W microwave power, 120°C.
Environmental Benefits :
- Reduces energy consumption by 40% compared to conventional heating.
- Avoids toxic solvents like diphenyl ether.
Comparative Analysis of Synthetic Methods
| Method | Steps | Key Reagents | Solvent | Temperature (°C) | Yield (%) | Scalability |
|---|---|---|---|---|---|---|
| Classical (1954) | 4 | SO2Cl2, NaNH3 | NH3(l) | -33 to 100 | 19 | Low |
| Na/Liq. NH3 (2005) | 2 | Boc-glutamic acid, Na/NH3 | THF | -33 | 55 | Moderate |
| Two-Step (2019) | 2 | Pyridine, ammonium acetate | Diphenyl ether | 170–175 | 56 | High |
| One-Pot (Patent) | 1 | Triethylamine, acetyl chloride | Toluene | 110–120 | 55–75 | Industrial |
| Microwave (2017) | 1 | DMAP | Solvent-free | 120 | 68 | Pilot-scale |
Mechanistic Insights and Side-Reaction Mitigation
Phthalimide Ring Formation
The nucleophilic attack of glutamic acid’s amino group on phthalic anhydride’s electrophilic carbonyl carbon initiates N-phthaloyl intermediate formation. Excess pyridine (2.5 eq) enhances reaction rates by absorbing liberated water.
Glutarimide Cyclization
Cyclization proceeds via intramolecular amide bond formation, facilitated by ammonium acetate’s ammonia release. Diphenyl ether’s high boiling point (259°C) enables reflux without solvent decomposition.
Side Reactions :
- Racemization : L-glutamic acid’s chiral center racemizes at >100°C, necessitating strict temperature control.
- Over-condensation : Excess phthalic anhydride leads to dimeric byproducts, mitigated by maintaining a 1:1.2 molar ratio (glutamic acid:phthalic anhydride).
Industrial Challenges and Solutions
Solvent Recovery
Diphenyl ether’s high cost ($450/kg) necessitates >90% recovery rates. Continuous distillation systems reduce solvent loss to <5% per batch.
Purification Simplification
Early methods required column chromatography, increasing production costs. Modern approaches use recrystallization from methanol/water mixtures, achieving >98% purity.
Q & A
Basic Research Questions
Q. How should clinical trials evaluating thalidomide’s efficacy in hematological malignancies be designed to account for refractory populations?
- Methodological Answer : Trials should incorporate dose-escalation protocols (e.g., starting at 200 mg/day, increasing by 200 mg every two weeks to 800 mg) and use paraprotein reduction (≥90% for complete response) as a primary endpoint, validated over at least six weeks . Survival metrics like event-free survival (Kaplan-Meier estimates) and stratification based on prior treatment history (e.g., post-high-dose chemotherapy relapse) are critical for ensuring clinical relevance. Bone marrow plasma cell counts and hemoglobin levels serve as secondary biomarkers .
Q. What in vitro models are suitable for studying this compound’s anti-angiogenic properties in vascular biology?
- Methodological Answer : Human coronary artery endothelial cells (HCAECs) can be treated with this compound dissolved in DMSO at varying concentrations (0.1–1.0 mM) to assess dose-dependent inhibition of proliferation and migration. Nano-CT imaging provides high-resolution quantification of neovascularization reduction in preclinical atherosclerosis models .
Q. How can cytokine modulation studies standardize measurements of this compound’s immunomodulatory effects?
- Methodological Answer : Use LPS-stimulated CD4+ T cells to quantify dose-dependent suppression of CXCR4/CCR5 expression (flow cytometry) and ELISA for IL-8/TNF-α levels in alveolar macrophages. Statistical validation via Wilcoxon tests for matched samples ensures robustness .
Advanced Research Questions
Q. How can contradictory data on this compound’s efficacy in cancer cachexia versus myeloma be reconciled?
- Methodological Answer : Heterogeneity in trial populations (e.g., frailty in cachexia vs. younger myeloma cohorts) and outcome definitions (weight stabilization vs. paraprotein reduction) must be addressed. Meta-analyses should stratify by disease stage and treatment history, while mechanistic studies (e.g., TNF-α pathway analysis) clarify context-dependent effects .
Q. What pharmacogenomic strategies identify patients at risk of this compound-associated venous thromboembolism (VTE)?
- Methodological Answer : Genome-wide SNP analysis (e.g., BOAC panel) prioritizes variants in MTHFR, Ku80, and Cyp3A5. Case-control studies nested within RCTs (e.g., MRC IX trial) match VTE cases to controls for allele frequency comparisons. Pathway-based analysis distinguishes tumor lysis-driven VTE (this compound) from inflammation-mediated events .
Q. Which molecular modeling approaches best predict this compound’s binding to cereblon (CRBN) for teratogenicity studies?
- Methodological Answer : AutoDock Vina simulations using CRBN crystallographic data (PDBID: 4CI1) validate binding modes via RMSD metrics (<1.5 Å). Multi-pose docking accounts for conformational flexibility, while quantum chemistry calculations assess chiral inversion dynamics in metabolites .
Q. How does minimal residual disease (MRD) status inform this compound maintenance therapy in myeloma?
- Methodological Answer : Multiparameter flow cytometry (MFC) detects MRD at 10⁻⁴ sensitivity post-ASCT. Stratify patients by cytogenetic risk (e.g., t(4;14)) and correlate MRD negativity with prolonged progression-free survival (PFS). Maintenance this compound’s efficacy is quantified by MRD conversion rates (28% in MRD-positive patients) .
Q. What experimental frameworks address this compound’s chiral inversion in pharmacokinetic studies?
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


