Etoposide
Description
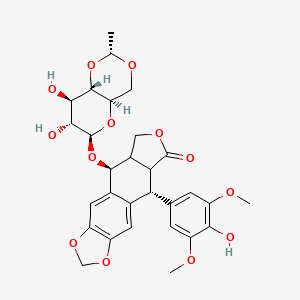
Etoposide (chemical formula: C₂₉H₃₂O₁₃) is a semisynthetic podophyllotoxin derivative and a topoisomerase II (Top2) inhibitor. It stabilizes Top2-DNA cleavage complexes, preventing DNA relegation and inducing double-strand breaks, leading to apoptosis . Clinically, this compound is used to treat small-cell lung cancer (SCLC), testicular cancer, and lymphomas, with response rates exceeding 80% in combination therapies .
Properties
Key on ui mechanism of action |
The drug appears to produce its cytotoxic effects by damaging DNA and thereby inhibiting or altering DNA synthesis. ... Etoposide appears to be cell-cycle dependent and cycle-phase specific, inducing G2 phase arrest and preferentially filling cells in the G2 and late S phases. Etoposide has been shown to arrest metaphase in chick fibroblasts, but its principal effect in mammalian cells appears to be in the G2 phase. At etoposide concentrations of 0.3-10 ug/ml in vitro, cells are inhibited from entering prophase; at concentrations of 10 ug/ml or higher, lysis of cells entering mitosis occurs. ... Etoposide does not inhibit microtubule assembly. Etoposide has been shown to induce single-stranded DNA breaks in HeLa cells and in murine leukemia L1210 cells in vitro; the drug also induces double-stranded DNA breaks and DNA-protein crosslinks in L1210 cells. Etoposide induced DNA damage appears to correlate well with the cytotoxicity of the drug. ... Etoposide appears to induce single-stranded DNA breaks indirectly, possibly through endonuclease activation, inhibition of intranuclear type II topoisomerase, or formation of a free-radical metabolite via an enzymatic reaction involving the hydroxyl group at the C-4' position of the E ring. Etoposide also reversibly inhibits the facilitated diffusion of nucleosides into HeLa cells in a concentration dependent manner in vitro. Etoposide may stabilize type II topoisomerase DNA complexes, preventing rejoining of single and double strand DNA breaks. Etoposide may also require cellular activation into intermediates, which then bind to DNA and disrupt cellular function. |
|---|---|
CAS No. |
33419-42-0 |
Molecular Formula |
C29H32O13 |
Molecular Weight |
588.6 g/mol |
IUPAC Name |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7S,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one |
InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25+,26-,27-,29+/m1/s1 |
InChI Key |
VJJPUSNTGOMMGY-QBUITQBFSA-N |
impurities |
The following impurities are limited by the requirements of The British Pharmacopoeia: 4'-carbenzoxy ethylidene lignan P, picroethylidene lignan P, alpha-ethylidene lignan P, lignan P and 4'-demethylepipodophyllotoxin. |
SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Isomeric SMILES |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Canonical SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Appearance |
White to off-white solid powder |
Color/Form |
Crystals from methanol |
melting_point |
236-251 °C |
Other CAS No. |
33419-42-0 |
physical_description |
Solid |
Pictograms |
Irritant; Health Hazard |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
>2 years if stored properly |
solubility |
Very soluble in methanol, chloroform; slightly soluble in ethanol, sparingly soluble in water. Sol in alc: approx 0.76 mg/ml Water solubility: approx 0.08 mg/mL |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
alpha-D-Glucopyranosyl Isomer Etoposide Celltop Demethyl Epipodophyllotoxin Ethylidine Glucoside Eposide Eposin Eto GRY Eto-GRY Etomedac Etopos Etoposide Etoposide Pierre Fabre Etoposide Teva Etoposide, (5a alpha)-Isomer Etoposide, (5a alpha,9 alpha)-Isomer Etoposide, (5S)-Isomer Etoposide, alpha D Glucopyranosyl Isomer Etoposide, alpha-D-Glucopyranosyl Isomer Etoposido Ferrer Farma Exitop Lastet NSC 141540 NSC-141540 NSC141540 Onkoposid Riboposid Teva, Etoposide Toposar Vépéside Sandoz Vépéside-Sandoz Vepesid VP 16 VP 16 213 VP 16-213 VP 16213 VP-16 VP16 |
vapor_pressure |
5.4X10-23 mm Hg at 25 °C /Estimated/ |
Origin of Product |
United States |
Preparation Methods
Etoposide is synthesized through a semisynthetic process starting from podophyllotoxin. One common method involves the direct condensation of 4’-demethyl epipodophyllotoxin with 2,3-di-O-dichloroacetyl-(4,6-O-ethylidene)-β-D-glucopyranose in the presence of trimethylsilyl trifluoromethane sulfonate (TMSOTf) to yield 4’-demethylepipodophyllotoxin-4-(2,3-di-O-dichloroacetyl-4,6-0-ethylidene)-β-D-glucopyranoside, which is then converted to this compound . This method provides enhanced yields, reduced reaction times, and more favorable isolation procedures compared to existing techniques .
Chemical Reactions Analysis
Etoposide undergoes several types of chemical reactions, including:
Reduction: Reduction reactions can convert this compound to its hydroquinone form.
Substitution: this compound can undergo substitution reactions, particularly at the glucopyranoside moiety.
Common reagents and conditions used in these reactions include oxidizing agents like hydrogen peroxide and reducing agents like sodium borohydride. The major products formed from these reactions include the O-quinone and hydroquinone derivatives of this compound .
Scientific Research Applications
Etoposide is a semi-synthetic derivative of podophyllotoxin that inhibits topoisomerase-II, leading to DNA strand breaks and apoptosis . Since its FDA approval in 1983, this compound has been used to treat solid and hematologic tumors, including lung cancer, germ cell tumors, and lymphoma .
This compound in Other Cancer Treatments
This compound, often in combination with other chemotherapeutic agents, is used to treat various cancers .
- Non-Hodgkin's Lymphoma this compound, combined with lomustine, methotrexate, and prednisone, is a first-line therapy for non-Hodgkin's lymphoma without major cardiotoxicity .
- Hodgkin's Disease this compound is a first-line chemotherapeutic agent combined with vincristine, chloramphenicol, and prednisolone, with a 77% response rate .
- Brain Tumors this compound has been observed to work against brain tumors in combination therapy with cyclophosphamide, cisplatin, vincristine, and carboplatin .
- Breast Cancer this compound has been explored in the management of metastatic breast cancer . A single-agent trial in untreated patients showed a response rate of approximately 15% .
- Germ Cell Tumors Adjuvant this compound plus cisplatin for 2 cycles has demonstrated prolonged disease-specific and relapse-free survival with acceptable toxicity and lower drug costs in patients with nonseminomatous germ cell tumors .
- Choriocarcinoma Low-dose this compound and cisplatin (EP) can be effective in treating elderly patients with choriocarcinoma .
Adverse Events and Toxicity
Mechanism of Action
Etoposide exerts its effects by inhibiting the enzyme topoisomerase II, which is responsible for regulating DNA topology through a double-strand passage mechanism . By forming a complex with topoisomerase II and DNA, this compound induces breaks in double-stranded DNA and prevents repair by topoisomerase II binding . Accumulated breaks in DNA prevent entry into the mitotic phase of cell division, leading to cell death . This compound acts primarily in the G2 and S phases of the cell cycle .
Comparison with Similar Compounds
Research Findings and Implications
- Selectivity: this compound’s Top2α preference and prolonged DNA damage underpin its clinical efficacy over non-selective inhibitors like genistein .
- Resistance Mechanisms : Mutations (e.g., Top2 Ser1106) and rapid DNA repair limit this compound efficacy, prompting combination therapies with natural compounds (e.g., quercetin) .
Biological Activity
Etoposide is a semisynthetic derivative of podophyllotoxin, primarily utilized as an antineoplastic agent in cancer therapy. Its biological activity is predominantly characterized by its ability to inhibit DNA topoisomerase II, leading to significant effects on DNA synthesis and cell cycle progression. This article delves into the mechanisms of action, clinical applications, and recent research findings regarding this compound's biological activity.
This compound exerts its effects primarily through the following mechanisms:
- Inhibition of DNA Topoisomerase II : this compound forms a complex with topoisomerase II, preventing the re-ligation of DNA strands after they have been cleaved. This results in the accumulation of DNA double-strand breaks (DSBs), which can trigger apoptosis in cancer cells .
- Cell Cycle Specificity : The drug is cell cycle-dependent, affecting mainly the S and G2 phases. At high concentrations, it can induce cell lysis during mitosis, while lower concentrations inhibit cells from entering prophase .
- Activation of DNA Damage Response : this compound activates key proteins involved in the DNA damage response, such as ATM (Ataxia Telangiectasia Mutated) kinase, which leads to the formation of repair foci and chromosomal abnormalities if DSBs are not repaired .
Clinical Applications
This compound is widely used in various chemotherapy regimens for different types of cancers, particularly small-cell lung cancer (SCLC) and testicular cancer. Its effectiveness can be influenced by the scheduling of administration:
- Small-Cell Lung Cancer : In a randomized trial comparing two administration schedules, the five-day infusion regimen demonstrated an overall response rate of 89%, significantly higher than the 10% observed with a 24-hour continuous infusion schedule .
- Combination Therapies : this compound is often combined with other agents. For instance, in treating extensive SCLC, combinations with cisplatin have shown objective response rates ranging from 44% to 78% .
Research Findings and Case Studies
Recent studies have expanded our understanding of this compound's biological activity beyond its traditional uses:
- Antibacterial Activity : A study explored this compound's antibacterial properties when combined with hydroxyapatite, suggesting potential repurposing for treating infections .
- Pediatric Oncology : The OLIE trial evaluated the combination of lenvatinib, ifosfamide, and this compound in children with high-grade osteosarcoma, showing promising outcomes .
- Toxicity Management : Research has indicated that this compound can cause myelosuppressive toxicity; however, recent trials have focused on optimizing dosing regimens to mitigate these effects while maintaining efficacy .
Data Summary
The following table summarizes key findings from various studies on this compound:
Q & A
Q. What is the primary mechanism by which etoposide exerts its anticancer effects?
this compound targets DNA topoisomerase II (Topo II), stabilizing the enzyme-DNA cleavage complex and preventing religation of DNA strands. This results in double-strand breaks (DSBs) and triggers apoptosis via activation of stress-associated signaling pathways (e.g., p53-mediated cell cycle arrest) . Notably, only 0.3% of this compound-induced strand breaks activate H2AX phosphorylation, suggesting most damage is non-toxic .
Q. How can researchers design experiments to assess this compound-induced cytotoxicity in vitro?
Standard protocols include measuring lactate dehydrogenase (LDH) release as a cell death marker, flow cytometry for cell cycle distribution (e.g., G2/M arrest), and apoptosis assays (Annexin V/PI staining). For example, SIN-1-treated astrocytes under glucose deprivation showed this compound's cytoprotective effect via LDH release quantification .
Q. What factors influence the variable oral bioavailability of this compound, and how can these be addressed preclinically?
this compound's low solubility (BCS Class IV) and rapid clearance (terminal half-life: 1.5 hours) limit bioavailability (24–74%). Researchers use nanosuspensions stabilized with surfactants (e.g., PLGA nanoparticles) and optimize formulations via Box–Behnken factorial designs to enhance dissolution and sustained release .
Advanced Research Questions
Q. How can experimental design mitigate biases in studies comparing this compound combination therapies (e.g., cisplatin + this compound)?
Rigorous protocols involve blinding, randomization, and standardized dosing. A meta-analysis of small-cell lung cancer trials highlighted the importance of controlling for confounders like patient stratification and toxicity metrics (e.g., myelosuppression rates) to validate efficacy claims .
Q. What molecular mechanisms underlie this compound resistance, and how can they be systematically investigated?
Resistance arises via reduced Topo II expression, overexpression of drug efflux pumps (ABCC1/ABCC6), or dysregulated signaling pathways (JAK-STAT, MAPK). Gene expression profiling (microarrays, RNA-seq) and functional assays (e.g., siRNA knockdown) in resistant cell lines (e.g., MCF7VP) reveal key pathways . Fluctuation analysis in MES-SA cells estimated mutation rates (2.9 × 10⁻⁶ to 1.7 × 10⁻⁷ per generation) for spontaneous resistance .
Q. How do contradictory findings on this compound's dual role in cytotoxicity and cytoprotection arise, and how can they be resolved?
this compound scavenges peroxynitrite (ONOO⁻) in glucose-deprived astrocytes, reducing oxidative stress independent of cell cycle inhibition . Contradictions may stem from model-specific variables (e.g., cell type, stress conditions). Researchers should validate mechanisms using multiple assays (e.g., glutathione depletion, superoxide anion measurement) .
Q. What advanced formulations improve this compound's pharmacokinetics, and how are they optimized?
Amorphous nanopowders (particle size: ~200 nm) using freeze-drying with cryoprotectants (mannitol) enhance oral absorption (Cmax: 2.21×; AUC: 2.13× vs. coarse powder). Optimization involves response surface methodology for ultrasonication time, phase ratios, and stabilizer concentration .
Methodological Guidance
Q. How should researchers statistically analyze survival data from this compound-treated cohorts?
Use Kaplan-Meier survival curves with log-rank tests for significance. A meta-analysis of high-grade glioma trials reported median survival gains (SG: 7–23 months) and stratified results by treatment regimen (e.g., VP-16 vs. VM-26) .
Q. What in vitro models best recapitulate this compound's blood-brain barrier penetration for CNS cancer studies?
Primary rat astrocyte cultures under glucose deprivation and oxidative stress (SIN-1 exposure) mimic the blood-brain barrier's metabolic constraints. Measure NO release with Clark-type electrodes and superoxide with lucigenin chemiluminescence .
Q. How can researchers validate this compound's target engagement in DNA damage assays?
Comet assays quantify DSBs/SSBs, while γ-H2AX immunofluorescence marks repair foci. Calicheamicin-induced DSBs serve as positive controls to distinguish Topo II-dependent damage .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


