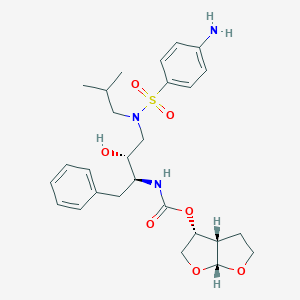
Darunavir
Description
Historical Context and Discovery Timeline
The discovery and development of this compound occurred within the broader context of protease inhibitor evolution, building upon decades of research into human immunodeficiency virus biochemistry and drug resistance mechanisms. The timeline begins with foundational work on protease inhibitors in the early 1990s, when the first compounds in this class, including saquinavir, established the therapeutic potential of targeting the viral protease enzyme. This initial success was followed by the development of ritonavir, indinavir, and nelfinavir throughout the mid-1990s, each contributing important insights into structure-activity relationships and resistance patterns.
The specific development of this compound traces back to collaborative research efforts involving the Belgian pharmaceutical company Tibotec, which recognized the need for more sophisticated approaches to overcome emerging resistance issues. Initial research phases focused on understanding the limitations of existing protease inhibitors and identifying structural modifications that could address these shortcomings. The compound was originally designated as UIC-94017 during early development phases, later becoming known as TMC-114 before receiving its final name this compound.
The timeline of this compound development reveals a systematic progression through various research phases, beginning with molecular design studies that identified the bis-tetrahydrofuran ligand as a key structural element. Clinical development commenced with POWER 1 and POWER 2 trials, which demonstrated the compound's efficacy in treatment-experienced patients who had limited therapeutic options. These studies provided crucial evidence supporting the compound's ability to maintain activity against resistant viral strains, leading to accelerated regulatory review processes.
The regulatory milestone occurred on June 23, 2006, when the United States Food and Drug Administration granted approval for this compound, marking its entry into clinical practice. This approval was initially limited to treatment-experienced patients, reflecting the compound's particular value in addressing resistant viral infections. Subsequent clinical studies, including the ARTEMIS trial, demonstrated efficacy in treatment-naive populations, leading to expanded approval indications in 2008. The European regulatory approval followed a similar timeline, with initial authorization in 2006 and subsequent expansions of approved uses.
| Development Milestone | Year | Significance |
|---|---|---|
| Initial Discovery Research | Early 2000s | Identification of bis-tetrahydrofuran ligand importance |
| Compound Designation UIC-94017 | 2003-2004 | Early development phase compound identification |
| TMC-114 Development | 2004-2005 | Advanced preclinical development and optimization |
| POWER 1 and 2 Clinical Trials | 2005-2006 | Clinical efficacy demonstration in treatment-experienced patients |
| United States Food and Drug Administration Approval | June 23, 2006 | Initial regulatory approval for treatment-experienced populations |
| European Union Approval | 2006 | Parallel regulatory approval in European markets |
| Expanded United States Approval | 2008 | Approval for treatment-naive populations |
| Generic Versions Available | 2018 | Patent expiration and generic market entry |
IUPAC Nomenclature and Structural Classification
The International Union of Pure and Applied Chemistry nomenclature for this compound reflects the compound's complex molecular architecture and stereochemical precision. The complete IUPAC name is [(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate. This comprehensive nomenclature captures the stereochemical complexity inherent in the molecule, with multiple chiral centers requiring precise stereochemical designation to ensure accurate identification and synthesis.
The structural classification of this compound places it within the broader category of protease inhibitors, specifically as a non-peptidic hydroxyethylamine derivative. This classification distinguishes it from earlier peptidic inhibitors while maintaining the essential hydroxyl group that mimics the transition state of peptide bond hydrolysis. The compound's structure incorporates several distinct molecular regions, each contributing specific functional properties to its overall activity profile.
The bis-tetrahydrofuran moiety represents one of the most significant structural innovations in this compound's design, serving as the P2 ligand in the enzyme active site. This bicyclic ether system provides optimal positioning for hydrogen bonding interactions with the enzyme backbone, contributing significantly to the compound's binding affinity and selectivity. Research has demonstrated that this structural element is crucial for maintaining activity against resistant viral strains, as it forms more extensive and stable interactions compared to the ligands found in earlier protease inhibitors.
The para-aminosulfonamide group functions as the P2' ligand, providing additional binding interactions and contributing to the compound's favorable pharmacokinetic properties. This structural element represents a departure from the methoxy groups found in some earlier compounds, offering improved water solubility and bioavailability characteristics. The isobutyl group attached to the sulfonamide nitrogen provides hydrophobic interactions that enhance binding affinity while maintaining appropriate molecular properties for oral bioavailability.
| Structural Component | Chemical Description | Functional Role |
|---|---|---|
| Bis-tetrahydrofuran moiety | Bicyclic ether system with defined stereochemistry | P2 ligand providing enzyme backbone hydrogen bonding |
| para-Aminosulfonamide group | Aromatic sulfonamide with primary amine | P2' ligand enhancing binding and solubility |
| Hydroxyethylamine core | Central hydroxyl-containing carbon chain | Transition state mimetic for enzyme binding |
| Isobutyl substituent | Branched alkyl chain | Hydrophobic binding enhancement |
| Phenyl groups | Aromatic ring systems | Hydrophobic interactions and molecular rigidity |
| Carbamate linkage | Ester-amide functional group | Structural connectivity and stability |
The molecular weight of this compound is 547.66 grams per mole, reflecting its substantial size and complexity compared to many small molecule therapeutics. The compound contains 27 carbon atoms, 37 hydrogen atoms, 3 nitrogen atoms, 7 oxygen atoms, and 1 sulfur atom, distributed across a three-dimensional structure that has been optimized for specific protein-ligand interactions. The presence of multiple heteroatoms provides numerous opportunities for hydrogen bonding and polar interactions, contributing to the compound's selectivity and binding strength.
Role in Antiretroviral Drug Development
This compound's emergence marked a pivotal moment in antiretroviral drug development, representing the culmination of lessons learned from first-generation protease inhibitors and the application of advanced medicinal chemistry principles to address persistent therapeutic challenges. The compound's development occurred during a critical period when clinicians were encountering increasing numbers of patients with multidrug-resistant viral infections, necessitating new approaches to treatment. This context shaped the design goals for this compound, emphasizing the need for activity against resistant strains while maintaining potent efficacy against wild-type virus.
The compound's role in advancing antiretroviral therapy extends beyond its individual therapeutic contributions to encompass its influence on drug design paradigms and resistance management strategies. Research demonstrating this compound's effectiveness against resistant viral strains provided crucial insights into the molecular basis of resistance and the structural requirements for overcoming such resistance. These findings influenced subsequent drug development efforts, establishing new standards for evaluating potential therapeutic candidates and informing structure-activity relationship studies across the field.
The development of this compound also contributed significantly to understanding the relationship between molecular structure and resistance patterns in protease inhibitors. Studies examining the compound's activity against panels of resistant viral strains revealed that certain structural features, particularly the bis-tetrahydrofuran moiety, were critical for maintaining activity against mutations that rendered other inhibitors ineffective. This research provided valuable guidance for designing future compounds and understanding the mechanistic basis of drug resistance in viral enzymes.
The compound's introduction into clinical practice demonstrated the value of targeting specific resistance mechanisms through rational drug design approaches. Clinical trials showed that this compound maintained significant activity in patients who had previously failed multiple treatment regimens, providing evidence that sophisticated molecular design could overcome resistance barriers that had limited earlier therapeutic options. These results validated the investment in advanced drug development approaches and encouraged continued research into next-generation antiretroviral agents.
Research examining this compound's mechanism of action has revealed insights into the molecular basis of protease inhibition and the factors that contribute to resistance development. Studies have shown that the compound's binding mode involves extensive hydrogen bonding interactions with the enzyme backbone, creating a network of contacts that are difficult for the virus to disrupt through mutation. This understanding has informed efforts to develop even more advanced compounds that can anticipate and prevent resistance development.
The influence of this compound on antiretroviral drug development extends to its impact on combination therapy strategies and treatment guidelines. The compound's favorable resistance profile and potent activity have made it a preferred component in many treatment regimens, influencing how clinicians approach therapy selection and resistance management. This clinical utility has provided valuable real-world experience with advanced protease inhibitors and has informed the development of treatment protocols that maximize therapeutic benefit while minimizing resistance risk.
| Development Impact | Specific Contribution | Broader Significance |
|---|---|---|
| Resistance Mechanism Understanding | Identification of structural features that prevent resistance | Informed design of subsequent antiretroviral compounds |
| Clinical Efficacy Demonstration | Activity in heavily treatment-experienced patients | Validated advanced drug design approaches |
| Molecular Design Principles | Bis-tetrahydrofuran ligand optimization | Established new paradigms for ligand design |
| Treatment Protocol Evolution | Integration into preferred regimen guidelines | Influenced clinical practice standards |
| Structure-Activity Relationships | Correlation between molecular features and resistance | Advanced understanding of drug-target interactions |
| Pharmaceutical Development | Advanced synthetic methodologies and formulation | Contributed to manufacturing and delivery technologies |
Properties
IUPAC Name |
[(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CJBJHOAVZSMMDJ-HEXNFIEUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2COC3C2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)CN(C[C@H]([C@H](CC1=CC=CC=C1)NC(=O)O[C@H]2CO[C@@H]3[C@H]2CCO3)O)S(=O)(=O)C4=CC=C(C=C4)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H37N3O7S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0046779 | |
| Record name | Darunavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0046779 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
547.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Approximately 0.15 mg/mL at, 6.68e-02 g/L | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
White, amorphous solid | |
CAS No. |
206361-99-1 | |
| Record name | Darunavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=206361-99-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Darunavir [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0206361991 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0046779 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Carbamic acid, N-[(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-, (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DARUNAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YO603Y8113 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Darunavir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7788 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Darunavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015393 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
74-76, 74 °C (decomposes) | |
| Record name | Darunavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01264 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Darunavir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7788 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
Condensation of Amino and Furanyl Intermediates
The primary route to Darunavir involves coupling (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol (furofuranol) with a sulfonamide-bearing amino compound. As detailed in patent US9062065B2, the amino compound [(1S,2R)-3-[(4-nitrophenyl)sulfonylamino]-2-hydroxy-1-(phenylmethyl)propyl]carbamate (4) reacts with a furanyl carbonate derivative (3) in dichloromethane under basic conditions. The reaction proceeds at ambient temperature for 48 hours, achieving a 1:1.2 molar ratio of amino compound to furanyl derivative to minimize side reactions. Solvent selection is critical: polar aprotic solvents like dichloromethane enhance coupling efficiency by stabilizing the transition state.
Nitro Group Reduction
The resulting nitro intermediate (5) undergoes catalytic hydrogenation using palladium on carbon (Pd/C) in ethanol. This step reduces the nitro group to an amine while preserving the stereochemical integrity of the molecule. Patent US8703980B2 reports a 92% yield for this reduction, with residual palladium levels below 10 ppm after filtration and recrystallization. Alternative reductants like sodium dithionite have been explored but exhibit lower selectivity.
Bicyclic Side Chain Synthesis from Monopotassium Isocitrate
Fermentation-Derived Starting Material
The stereochemically complex furofuranol side chain is synthesized from monopotassium isocitrate, a fermentation product of Aspergillus niger fed with sunflower oil. This biobased approach ensures a sustainable and cost-effective supply chain, with raw material costs estimated at $50/kg.
Key Reduction and Cyclization Steps
Isocitrate is converted to a tertiary N-methylaniline amide (5b) to facilitate lithium aluminum hydride (LAH) reduction. LAH selectively reduces ester and amide functionalities to generate a triol intermediate, which spontaneously cyclizes under acidic conditions to form the furofuranol structure. This one-pot process achieves a 78% yield for the bicyclic product, with diastereomeric purity exceeding 99.8% after crystallization.
Table 1: Optimization of LAH Reduction
| Parameter | Value | Impact on Yield |
|---|---|---|
| LAH Equivalents | 4.5 | Maximizes conversion |
| Temperature | 0°C → 23°C (gradual) | Prevents overreduction |
| Quenching Method | Aqueous HCl (pH 2) | Stabilizes triol |
This compound Ethanolate Particle Engineering
Milling for Controlled Particle Size
Post-synthesis, this compound ethanolate is processed to achieve particle sizes critical for bioavailability. Patent US9062065B2 describes a nitrogen jet milling technique where coarse powder is fed into a rotating chamber at 20–30 rpm under 2–3 kg/cm² pressure. Centrifugal forces segregate particles by size, with fines (<10 μm) collected via central exhaust. This method reduces d₀.₉ (90th percentile) from 250 μm to 130 μm, ensuring uniform dissolution.
Table 2: Particle Size Distribution of Milled Ethanolate
| Metric | Pre-Milling (μm) | Post-Milling (μm) |
|---|---|---|
| d₀.₉ | 250 | 130 |
| d₀.₅ | 75 | 27 |
| d₀.₁ | 20 | 8 |
Crystallization and Polymorph Control
Ethanolate crystallization from ethanol/water mixtures (9:1 v/v) yields the thermodynamically stable Form I polymorph. X-ray diffraction confirms a monoclinic crystal system with hydrogen bonding between ethanol and the sulfonamide group, critical for long-term stability.
Comparative Analysis of Synthetic Routes
Cost and Yield Considerations
The isocitrate-based side chain synthesis reduces production costs by 33% compared to traditional chemical routes, primarily due to lower raw material expenses. However, the condensation-reduction method remains dominant in industrial settings due to its scalability and familiarity.
Chemical Reactions Analysis
Types of Reactions: Darunavir undergoes various chemical reactions, including oxidation, reduction, and substitution. It is heavily oxidized and metabolized by hepatic cytochrome enzymes, primarily CYP3A4 .
Common Reagents and Conditions:
Oxidation: Involves hepatic cytochrome enzymes.
Reduction: Not commonly reported for this compound.
Substitution: Involves reactions with carbonates and amines.
Major Products: The major products formed from these reactions include hydroxylated and glucuronidated metabolites .
Scientific Research Applications
Efficacy in Treatment-Naïve Patients
A meta-analysis involving multiple studies has shown that darunavir/ritonavir-based regimens are effective for treatment-naïve patients. The virological response rate was comparable to other first-line therapies:
| Study | Population | Response Rate | |
|---|---|---|---|
| ARTEMIS Trial | Treatment-Naïve | 84% at 48 weeks | Effective compared to other ARTs |
| POWER Trials | Treatment-Experienced | 73% at 48 weeks | Superior virological response |
The ARTEMIS trial specifically highlighted that this compound/ritonavir was effective in achieving viral suppression in treatment-naïve patients over a 48-week period .
Efficacy in Treatment-Experienced Patients
This compound has shown significant efficacy in treatment-experienced patients, particularly those with prior exposure to other antiretroviral agents. A study indicated that this compound/ritonavir provided a higher virological response compared to alternative treatments:
| Study | Population | Response Rate | |
|---|---|---|---|
| This compound Outcomes Study | Three-Class Experienced | 63% at 48 weeks | Demonstrated superior efficacy over other regimens |
| GRACE Study | Sex-Based Outcomes | No significant differences between sexes | Effective across genders |
The this compound Outcomes Study confirmed that patients with multiple drug resistance had a sustained virological response when treated with this compound .
Safety Profile
This compound is generally well-tolerated, with adverse events reported at similar rates compared to other antiretrovirals. Common side effects include gastrointestinal symptoms and rash, but serious adverse events are rare:
| Adverse Event | Frequency (%) |
|---|---|
| Diarrhea | 10 |
| Rash | 7 |
| Nausea | 5 |
In clinical trials, this compound's safety profile was comparable to other antiretroviral therapies, reinforcing its use as a first-line agent .
Innovative Research and Future Directions
Recent studies have focused on developing this compound analogs using advanced computational methods such as fragment molecular orbital techniques. These efforts aim to create compounds with enhanced efficacy against resistant HIV strains:
- FMO-guided Design : Novel this compound analogs are being developed to overcome resistance issues associated with HIV-1 protease mutations.
- Molecular Docking Studies : These studies assess the binding affinity of new analogs against wild-type and mutant strains of HIV-1 protease.
The ongoing research aims to improve the therapeutic options available for patients who exhibit resistance to current treatments .
Case Studies
-
Case Study: Treatment-Naïve Patient
- Background : A 35-year-old male diagnosed with HIV-1.
- Treatment : Initiated on this compound/ritonavir plus two nucleoside reverse transcriptase inhibitors.
- Outcome : Achieved viral load suppression (<50 copies/mL) by week 48.
-
Case Study: Treatment-Experienced Patient
- Background : A 50-year-old female with extensive treatment history and resistance mutations.
- Treatment : Switched to this compound/ritonavir.
- Outcome : Achieved sustained viral suppression after 24 weeks despite previous failures on other regimens.
Mechanism of Action
Darunavir exerts its effects by inhibiting the HIV-1 protease enzyme, which is essential for viral precursor protein processing and viral maturation. By binding to the enzyme’s active site, this compound prevents the cleavage of the HIV Gag-Pol polyprotein, thereby blocking the formation of infectious virions . This mechanism is crucial for its antiretroviral activity.
Comparison with Similar Compounds
Comparison with Similar Compounds
Structural and Mechanistic Comparison
Darunavir’s structural innovations differentiate it from other PIs:
| Compound | Generation | Key Structural Features | Mechanism of Action | Resistance Barrier |
|---|---|---|---|---|
| This compound | Second-gen | Bis-THF, sulfonamide isostere | 8 hydrogen bonds with protease active site | High |
| Amprenavir | First-gen | Hydroxyethylamine sulfonamide | 4 hydrogen bonds | Moderate |
| Tipranavir | Second-gen | Dihydropyrone, sulfonamide | Non-peptidic binding | Moderate-High |
| Saquinavir | First-gen | Peptidomimetic, hydroxyethylamine isostere | Substrate analog | Low |
- Amprenavir : this compound’s parent compound. This compound’s bis-THF group increases hydrogen bonding (8 vs. 4 bonds), improving binding affinity (IC₅₀: 0.1 nM vs. 0.6 nM) .
- Tipranavir: A non-peptidic PI with a distinct dihydropyrone scaffold. While effective against some this compound-resistant strains, it has higher IC₅₀ (2.8 nM) and requires thrice-daily dosing .
- Saquinavir : First-generation peptidomimetic PI. Susceptibility drops significantly with mutations (e.g., G48V, L90M), whereas this compound retains activity against many PI-resistant variants .
Resistance Profiles and Cross-Resistance
This compound’s high genetic barrier necessitates multiple mutations (e.g., V32I, I47V, I54M) for resistance. Cross-resistance patterns vary:
- Discrepancy in Data : One study reported only 28% of tipranavir-resistant isolates remained susceptible to this compound, highlighting variability in resistance testing methodologies .
Pharmacokinetics and Dosing
| Compound | Booster Required | Food Effect | Half-Life (h) | Dosing Frequency |
|---|---|---|---|---|
| This compound | Ritonavir/cobicistat | Yes (tablet) | 15 | Once/twice daily |
| Lopinavir | Ritonavir | No | 5–6 | Twice daily |
| Atazanavir | Ritonavir | Yes | 7–11 | Once daily |
- Cobicistat : A CYP3A4 inhibitor alternative to ritonavir. Unlike ritonavir, it lacks antiviral activity but reduces this compound’s pill burden .
Clinical Trial Outcomes
- POWER Trials : this compound + ritonavir achieved 70% viral suppression (<50 copies/mL) at 48 weeks in multidrug-resistant patients .
- ARTEMIS Study: this compound/ritonavir demonstrated non-inferiority to lopinavir/ritonavir in treatment-naïve patients (84% vs. 78% suppression) .
- DUAL Study : this compound/lamivudine maintained virologic suppression in 93% of patients switching from three-drug regimens .
Biological Activity
Darunavir (DRV) is a second-generation protease inhibitor (PI) used in the treatment of HIV-1 infection. It exhibits potent antiviral activity and is particularly effective against both wild-type and drug-resistant strains of the virus. This article delves into the biological activity of this compound, including its mechanisms of action, efficacy in clinical settings, and pharmacological properties, supported by relevant case studies and research findings.
This compound works by inhibiting the HIV-1 protease enzyme, which is crucial for the viral replication process. By binding to the active site of the protease, this compound prevents the cleavage of viral polyproteins into functional proteins, thereby halting viral maturation and replication.
Structural Insights
Recent studies have provided insights into this compound's binding interactions with HIV-1 protease. Modifications in its structure have been shown to enhance its binding affinity through improved hydrogen bonding interactions within the enzyme's active site. For instance, modifications to the P2’ 4-amino group have led to derivatives with enhanced enzyme inhibitory activity .
Efficacy in Clinical Settings
This compound has been extensively studied for its efficacy in both treatment-naïve and treatment-experienced patients. A meta-analysis encompassing multiple randomized controlled trials revealed significant findings:
- For Treatment-Naïve Patients : this compound/ritonavir (DRV/r) demonstrated comparable virological response rates to other treatments at both 48 and 96 weeks, with no significant differences noted in efficacy .
- For Treatment-Experienced Patients : DRV/r showed a significantly higher virological response rate compared to other regimens (Risk Ratio: 1.45), indicating its effectiveness in patients who had previously failed therapy .
Pharmacokinetics and Safety Profile
This compound is primarily metabolized by CYP3A4 enzymes in the liver. Its pharmacokinetic profile allows it to be used effectively even in patients with varying degrees of liver function. Notably, a case study reported an unintentional overdose of this compound in an adolescent patient which resulted in minimal toxicity yet improved virological suppression, suggesting that higher doses may be beneficial under certain circumstances .
Table 1: Summary of Pharmacokinetic Properties
| Property | Value |
|---|---|
| Half-life | 15 hours |
| Bioavailability | ~37% |
| Peak plasma concentration | 2-4 hours post-dose |
| Volume of distribution | ~100 L |
Case Studies
- High-Dose this compound Use : An adolescent with reduced susceptibility to this compound underwent a regimen involving a high dose (1200 mg) which resulted in sustained viral load suppression (<400 copies/mL) and normalization of CD4 counts over a 24-month period .
- Meta-Analysis Findings : In a comprehensive review involving over 6,000 patients, DRV/r was found to be effective and well-tolerated across diverse populations, reinforcing its role as a frontline therapy for HIV .
Resistance and Future Directions
Emerging multidrug-resistant strains of HIV pose challenges to treatment efficacy. Research into this compound analogs aims to overcome these limitations by enhancing binding affinity and efficacy against resistant strains . Continuous monitoring of cardiovascular risks associated with this compound therapy is also essential, as some studies have indicated potential adverse effects on cardiovascular health .
Q & A
Q. How does darunavir’s molecular design confer a high genetic barrier to resistance compared to earlier protease inhibitors (PIs)?
this compound was designed using structural biology principles to preemptively address common resistance mutations. Its bis-tetrahydrofuranylurethane (bis-THF) group and sulfonamide moiety form extensive hydrogen bonds with conserved regions of the HIV-1 protease, reducing susceptibility to mutations. Unlike first-generation PIs, this compound’s scaffold minimizes steric clashes with mutated protease variants, maintaining binding affinity even when 18–21 resistance-associated mutations (RAMs) accumulate . Methodological Insight: Use X-ray crystallography and enzyme kinetics to compare this compound-protease binding patterns against mutant vs. wild-type proteases .
Q. What pharmacokinetic (PK) models are used to optimize this compound dosing in special populations (e.g., pregnant women)?
Semi-mechanistic population PK models integrating total and unbound this compound concentrations are critical. For pregnant women, nonlinear protein binding (due to increased α-1-acid glycoprotein) necessitates adjusted dosing. A study analyzing 2,601 plasma samples from 85 women used simulations to predict AUC0-τ and Ctrough during the third trimester, recommending therapeutic drug monitoring (TDM) to maintain unbound this compound levels above the protein-adjusted IC50 (PA-IC50) of 55 ng/mL .
Q. Which mutations are most strongly associated with reduced this compound susceptibility in treatment-experienced patients?
Primary RAMs include V32I, L33F, I47V, I54L/M, and L76V. Pooled data from POWER and DUET trials show that ≥3 RAMs reduce virologic response by 41% (vs. 78% in RAM-free strains). Genotypic resistance scores (e.g., ≥10 mutations from the IAS-USA list) correlate with phenotypic fold-changes in EC50 . Methodological Insight: Use phenotypic susceptibility assays (e.g., Antivirogram®) alongside genotypic sequencing to quantify resistance thresholds .
Advanced Research Questions
Q. How do structural dynamics in this compound-resistant protease variants impair inhibitor binding despite retained catalytic activity?
Highly mutated proteases (e.g., with 18–21 substitutions) exhibit distorted hydrogen-bond networks in the P2′ pocket, reducing this compound’s binding affinity by 20-fold. However, catalytic efficiency remains ~5% of wild-type levels, sufficient for viral replication. Crystallographic studies reveal that mutations like I84V shift the this compound aminophenyl group, disrupting key van der Waals interactions . Experimental Design: Combine cryo-EM for conformational analysis with molecular dynamics simulations to map residue flexibility .
Q. Why do some clinical trials show no correlation between this compound PK exposure and virologic suppression in susceptible strains?
In PI-naive patients, this compound/ritonavir 800/100 mg once-daily achieved similar AUCs to 600/100 mg twice-daily, but virologic responses were comparable (74–78% at 24 weeks). This suggests that exceeding the PA-IC50 threshold—not AUC maximization—is critical. Subanalyses indicate that adherence and baseline viral load are stronger predictors of outcome than PK variability . Data Contradiction Resolution: Apply multivariate regression to isolate PK parameters from confounding variables (e.g., adherence logs, baseline resistance) .
Q. What analytical methods validate this compound-ritonavir coformulations in bioequivalence studies?
- HPLC: A validated method with retention times of 2.358 min (this compound) and 3.099 min (ritonavir), resolution >2, and tailing factor <2% ensures specificity. System suitability testing requires ≤15% variability in peak area ratios .
- Spectrophotometry: A QBD-optimized fluorometric method using 0.1 N HCl achieves 98.15% recovery with ≤2% RSD in intraday/interday precision. Calibration curves are linear (5–30 μg/mL) with LOD/LOQ of 0.12/0.37 μg/mL .
Q. How can this compound resistance pathways inform the design of next-generation PIs?
Resistance to this compound requires accumulation of “clusters” of mutations (e.g., V32I + L33F + I47V + I54L). Structural modeling of these clusters reveals compensatory mechanisms (e.g., L76V restores protease stability). Novel PIs targeting the flap or hinge regions (e.g., GRL-0202) are designed to avoid these adaptive networks . Methodological Insight: Use deep mutational scanning to identify epistatic interactions between mutations .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


