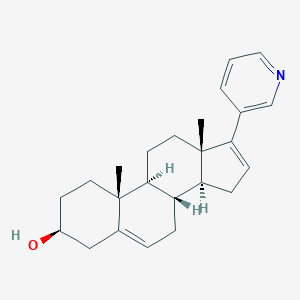
Abiraterone
Description
This compound is a potent, irreversible, and selective inhibitor of 17 αhydroxylase/C17,20-lyase (CYP17), an enzyme expressed in testicular, adrenal, and prostatic tumour tissues, to regulate androgen biosynthesis. This compound was first approved by the FDA and EMA on April, July, and September 2011, respectively. It is used to treat metastatic castration-resistant prostate cancer and hormone-sensitive high-risk metastatic prostate cancer. As this compound has poor oral bioavailability and is susceptible to hydrolysis by esterases, this compound acetate was developed as an orally bioavailable prodrug with enhanced stability and absorption.
This compound is a Cytochrome P450 17A1 Inhibitor. The mechanism of action of this compound is as a Cytochrome P450 17A1 Inhibitor, and Cytochrome P450 2D6 Inhibitor, and Cytochrome P450 2C8 Inhibitor.
This compound is a Cytochrome P450 17A1 Inhibitor. The mechanism of action of this compound is as a Cytochrome P450 17A1 Inhibitor.
This compound is a steroidal antiandrogen used to treat metastatic, castration-resistant prostate cancer. This compound is associated with an appreciable rate of serum enzyme elevation during therapy and with rare but potentially severe instances of acute liver injury with jaundice.
This compound is a steroidal compound with antiandrogen activity. This compound inhibits the enzymatic activity of steroid 17alpha-monooxygenase (17alpha-hydrolase/C17,20 lyase complex; CYP17A1), a member of the cytochrome p450 family that catalyzes the 17alpha-hydroxylation of steroid intermediates involved in testosterone synthesis. Administration of this agent may suppress testosterone production by both the testes and the adrenals to castrate-range levels.
See also: this compound Acetate (active moiety of).
Properties
IUPAC Name |
(3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GZOSMCIZMLWJML-VJLLXTKPSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC12CCC(CC1=CCC3C2CCC4(C3CC=C4C5=CN=CC=C5)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@]12CC[C@@H](CC1=CC[C@@H]3[C@@H]2CC[C@]4([C@H]3CC=C4C5=CN=CC=C5)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H31NO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID80879993 | |
| Record name | Abiraterone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80879993 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
349.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
154229-19-3 | |
| Record name | Abiraterone | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=154229-19-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Abiraterone [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0154229193 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Abiraterone | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05812 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Abiraterone | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80879993 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3-yl)tetracyclo[8.7.0.0²,�.0¹¹,¹�] heptadeca-7,13-dien-5-ol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ABIRATERONE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/G819A456D0 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Mechanistic Investigations of Abiraterone Action
Cytochrome P450 17A1 (CYP17A1) Inhibition by Abiraterone
Specificity of this compound as an Irreversible Inhibitor
This compound is characterized as a potent, selective, and irreversible inhibitor of CYP17A1 mdpi.compatsnap.comascopubs.org. Its mechanism involves binding to the heme iron of CYP17A1 via the nitrogen atom in its pyridine ring, forming a coordinate covalent bond mdpi.com. This interaction leads to a slow-binding inhibition, adhering to a two-step induced-fit mechanism nih.gov. While highly selective for CYP17A1, this compound has also been shown to inhibit other cytochrome P450 enzymes, such as CYP21A2, CYP1A2, CYP2D6, CYP3A4, CYP2C8, and CYP2C9 drugbank.comacs.org. This off-target inhibition may contribute to some of the observed side effects drugbank.comacs.org.
Impact on Steroidogenesis Pathways and Androgen Biosynthesis
The CYP17A1 enzyme possesses two crucial activities: 17α-hydroxylase and 17,20-lyase patsnap.comoup.comresearchgate.netnih.gov. This compound's inhibition of these activities profoundly impacts steroidogenesis:
17α-Hydroxylase Inhibition: This activity is essential for the conversion of pregnenolone to 17-hydroxypregnenolone and progesterone to 17-hydroxyprogesterone. These intermediates are critical precursors for cortisol synthesis patsnap.comoup.comnih.gov. By inhibiting this step, this compound leads to a significant decrease in cortisol production patsnap.comascopubs.orgoup.comnih.govnih.govresearchgate.netnih.govoup.com.
17,20-Lyase Inhibition: This activity is responsible for converting 17α-hydroxypregnenolone to dehydroepiandrosterone (DHEA) and 17α-hydroxyprogesterone to androstenedione. DHEA and androstenedione are then further converted to testosterone and other androgens mdpi.compatsnap.comoup.comnih.gov. This compound's blockade of this step effectively reduces the synthesis of androgens, including testosterone, DHEA, and androstenedione mdpi.compatsnap.comoup.comnih.govspandidos-publications.comnih.govnih.gov.
The inhibition of CYP17A1 leads to an accumulation of steroid precursors upstream of the blocked steps. Specifically, deoxycorticosterone (DOC) and corticosterone levels can increase significantly, with reported fold increases of approximately 10-fold and 40-fold, respectively researchgate.net. There is also a reported ~4-fold increase in 11-deoxycortisol researchgate.net.
Table 1: Changes in Steroid Precursor Levels Following this compound Inhibition of CYP17A1
| Steroid Precursor | Reported Change (Fold) | Supporting Source(s) |
| Deoxycorticosterone (DOC) | ~10-fold increase | researchgate.net |
| Corticosterone | ~40-fold increase | researchgate.net |
| 11-Deoxycortisol | ~4-fold increase | researchgate.net |
Downstream Molecular Effects of this compound on Hormone Production
Modulation of Adrenocorticotropic Hormone (ACTH) Axis by this compound
The reduction in cortisol synthesis caused by this compound's inhibition of CYP17A1 disrupts the negative feedback mechanism on the hypothalamic-pituitary-adrenal (HPA) axis patsnap.comascopubs.orgoup.comnih.govnih.govresearchgate.netnih.govnih.gov. Normally, adequate cortisol levels signal the pituitary gland to reduce the secretion of adrenocorticotropic hormone (ACTH). With decreased cortisol, this negative feedback is lost, leading to a compensatory surge in ACTH levels patsnap.comascopubs.orgoup.comnih.govnih.govresearchgate.netnih.govnih.govmdpi.comendocrine-abstracts.org. The elevated ACTH, in turn, further stimulates the adrenal glands to produce more steroid precursors, exacerbating the imbalance in steroidogenesis patsnap.comoup.comresearchgate.netnih.gov. The co-administration of glucocorticoids, such as prednisone, is intended to provide negative feedback to the pituitary, thereby suppressing this ACTH surge and mitigating the downstream effects oup.comresearchgate.netnih.govnih.govmdpi.comendocrine-abstracts.orgkarger.comprostatecancerpatientvoices.comresearchgate.net.
Alterations in Cortisol and Mineralocorticoid Levels Induced by this compound
This compound's inhibition of CYP17A1 leads to a dual effect on cortisol and mineralocorticoid levels:
Mineralocorticoid Excess: The elevated ACTH levels, driven by cortisol deficiency, stimulate the adrenal glands. The steroidogenic pathway is shunted towards the production of mineralocorticoid precursors, such as DOC and corticosterone, which are not subject to CYP17A1 inhibition patsnap.comascopubs.orgoup.comnih.govresearchgate.netnih.govmdpi.comnih.gov. These accumulated precursors exhibit mineralocorticoid activity, leading to an imbalance characterized by Mineralocorticoid Excess Syndrome (MES) drugbank.comoup.comnih.govresearchgate.netnih.govmdpi.comendocrine-abstracts.orgkarger.comprostatecancerpatientvoices.comnih.govoup.comdrugbank.comoup.com. MES typically manifests clinically as hypertension, hypokalemia, and fluid retention drugbank.comoup.comnih.govresearchgate.netnih.govendocrine-abstracts.orgkarger.comprostatecancerpatientvoices.comnih.govoup.comdrugbank.comoup.com. In some instances, this disruption of steroidogenesis can also lead to secondary adrenal insufficiency, particularly during times of stress or if glucocorticoid co-therapy is inadequate mdpi.comkarger.compreprints.org.
Compound Names Mentioned:
this compound
this compound acetate
Prednisone
Prednisolone
Dexamethasone
Cortisol
Corticosterone
Deoxycorticosterone (DOC)
11-Deoxycortisol
Progesterone
Pregnenolone
17-hydroxypregnenolone
17-hydroxyprogesterone
Dehydroepiandrosterone (DHEA)
Androstenedione
Testosterone
DHEA-S (Dehydroepiandrosterone sulfate)
Aldosterone
Estradiol
Estrone
Estrone sulfate
Pharmacodynamic Characterization of Abiraterone
Androgen Receptor Signaling Modulation by Abiraterone
This compound's principal pharmacodynamic action is the inhibition of the cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) enzyme patsnap.comscispace.comnih.govoup.com. CYP17A1 is essential for the synthesis of androgens, including testosterone, in the adrenal glands, testes, and prostate tumor tissues patsnap.comoup.comfda.gov. This compound functions as a selective and irreversible inhibitor of this enzyme, binding to its heme iron and thereby blocking its catalytic activity patsnap.comscispace.comnih.govoup.comfda.govnih.gov. This inhibition prevents the conversion of pregnenolone and progesterone into 17α-hydroxy derivatives and subsequently blocks the formation of dehydroepiandrosterone (DHEA) and androstenedione, the precursors to testosterone patsnap.comoup.com.
The resultant significant reduction in androgen levels is central to this compound's modulation of androgen receptor (AR) signaling patsnap.comscispace.com. Prostate cancer cells typically rely on androgens to bind to AR, activating downstream pathways that promote cell proliferation and inhibit apoptosis patsnap.combioscientifica.com. By diminishing the availability of these ligands, this compound effectively decreases AR activation, thereby limiting the pro-growth signals within cancer cells patsnap.com. This mechanism is particularly crucial in castration-resistant prostate cancer (CRPC), where tumors often develop mechanisms to sustain AR signaling despite low circulating testosterone levels, including intratumoral androgen synthesis fda.govbioscientifica.com. This compound's ability to inhibit androgen production at multiple sites helps to overcome this resistance nih.govoup.comfda.gov.
However, AR signaling can persist or adapt in the presence of this compound through various mechanisms, such as AR gene amplification, mutations leading to increased AR expression or ligand-independent activation, or the emergence of AR splice variants like AR-V7 bioscientifica.commdpi.comoncotarget.comaacrjournals.org. Some AR mutations, such as AR-T877A, can even lead to resistance by allowing cells to be activated by other steroids, like progesterone, whose levels may increase due to CYP17A1 inhibition oncotarget.com.
Cellular and Molecular Responses to this compound Treatment
This compound treatment elicits a range of cellular and molecular responses that contribute to its antitumor activity.
Cell Growth and Proliferation: this compound demonstrably attenuates cell growth. In androgen receptor (AR)-positive LNCaP cells, this compound treatment significantly decreased cell growth, alongside reductions in AR expression and activity spandidos-publications.com. Similarly, AR-negative PC-3 cells exhibited comparable reductions in cellular proliferation when treated with this compound, suggesting effects beyond direct AR blockade spandidos-publications.com. For instance, PC-3 cells treated with 30 μM this compound for 120 hours showed reduced proliferation spandidos-publications.com. Furthermore, this compound's modulation of the osteoblast secretome led to reduced cancer cell proliferation in AR-dependent C4-2B cells (p = 0.022) and a significant reduction in AR activation (p = 0.017) nih.gov.
Apoptosis and Molecular Pathway Modulation: this compound can induce apoptosis and modulate apoptotic pathways, evidenced by changes in key proteins such as p21, caspase-3, survivin, and transforming growth factor β (TGFβ) spandidos-publications.com. Some studies indicate that this compound may downregulate the anti-apoptotic protein BCL2 and upregulate the pro-apoptotic protein BAX, thereby activating apoptosis pathways researchgate.net.
Gene Expression and Signaling: this compound treatment can induce global gene expression changes nih.gov. It has been observed to increase intracellular cAMP levels and induce kinase activity, leading to the phosphorylation of CREB1 (pCREB1) nih.govaacrjournals.org. This upregulation of pCREB1 is critical for controlling global gene expression and has been implicated in the development of this compound resistance, potentially by augmenting the function of the CBP/p300 complex nih.govaacrjournals.org.
Metabolite Activity: A metabolite of this compound, 3-keto-5α-abiraterone, shares structural similarities with androgens and can activate pro-cancer pathways by tricking androgen receptors, potentially fueling cancer cell growth clevelandclinic.org.
Data Tables
Table 1: Summary of this compound's Mechanism of CYP17A1 Inhibition
| Target Enzyme | Enzyme Function | Inhibition Type | Key Outcome | Reference(s) |
| CYP17A1 | 17α-hydroxylase and 17,20-lyase | Selective, irreversible, slow-tight binding | Reduced androgen biosynthesis (testosterone, DHEA, androstenedione) | patsnap.comscispace.comnih.govoup.comfda.govnih.gov |
| CYP17A1 | Heme component | Coordination of pyridine ring with heme iron | Potent inhibition of catalytic activity. High-affinity complex Ki* = 0.39 nM | oup.comnih.gov |
Table 2: Cellular and Molecular Responses to this compound Treatment (Examples)
| Cell Line | Treatment | Effect | Key Finding/Metric | Reference(s) |
| LNCaP | This compound | Decreased cell growth | Significant reduction | spandidos-publications.com |
| LNCaP | This compound | Decreased AR expression | Significant reduction | spandidos-publications.com |
| LNCaP | This compound | Decreased AR activity | Significant reduction | spandidos-publications.com |
| PC-3 | This compound (30 μM, 120h) | Attenuated cell growth | Comparable reduction to AR+ cells | spandidos-publications.com |
| C4-2B | Osteoblast conditioned media + this compound | Reduced cancer cell proliferation | p = 0.022 | nih.gov |
| C4-2B | Osteoblast conditioned media + this compound | Reduced AR activation | p = 0.017 | nih.gov |
| Prostate cancer cells | This compound treatment | Increased phosphorylated CREB1 (pCREB1) | Critical for global gene expression | nih.govaacrjournals.org |
| Prostate cancer cells | This compound treatment | Modulation of apoptotic proteins (e.g., BCL2, BAX) | Induction of apoptosis pathways | spandidos-publications.comresearchgate.net |
Pharmacokinetic Profiles of Abiraterone and Its Metabolites
Prodrug Conversion of Abiraterone Acetate to this compound
Following oral administration, this compound acetate is rapidly and extensively converted to this compound. europa.eumdpi.com This conversion is a hydrolysis reaction, catalyzed by esterases, though the specific esterases involved have not been fully identified. mdpi.comdrugbank.comnih.gov This process occurs pre-systemically, meaning it largely takes place before the drug enters the main circulation. mdpi.com In clinical studies, plasma concentrations of the prodrug, this compound acetate, are typically undetectable in the vast majority of samples. drugbank.com The rapid hydrolysis in the intestinal environment can lead to the generation of this compound concentrations that exceed its normal solubility, a phenomenon known as intestinal supersaturation, which creates a strong driving force for its absorption. nih.gov
Hepatic Metabolism of this compound and its Metabolites
This compound undergoes extensive metabolism, primarily in the liver. europa.eu The biotransformation pathways include sulfation, hydroxylation, and oxidation. europa.eu The cytochrome P450 enzyme system, particularly CYP3A4, plays a significant role in its metabolism. nih.govcancercareontario.ca
Several key metabolites of this compound have been identified, which result from its complex metabolic pathways.
Δ4-Abiraterone (D4A): This metabolite is formed from this compound through the action of the enzyme 3β-hydroxysteroid dehydrogenase (3βHSD). prostatecancertopics.comnih.gov D4A is considered a more potent antitumor agent than this compound itself. prostatecancertopics.comaacrjournals.org
5α-Abiraterone: Δ4-abiraterone can be further metabolized by 5α-reductase to form 3-keto-5α-abiraterone, also known as 5α-abiraterone. wikipedia.orgoaepublish.com This metabolite has been found to have androgenic activity, potentially counteracting the therapeutic effect of this compound. wikipedia.org
This compound Sulfate and N-oxide this compound Sulfate: These are two major circulating, but pharmacologically inactive, metabolites found in human plasma. drugbank.comnih.gov Together, they account for approximately 86% of the drug's exposure. drugbank.com The formation of this compound sulfate involves the SULT2A1 enzyme, while N-oxide this compound sulfate formation involves both CYP3A4 and SULT2A1. drugbank.comnih.gov
Table 1: Key Metabolites of this compound
| Metabolite | Precursor | Key Enzyme(s) | Pharmacological Activity |
|---|---|---|---|
| Δ4-Abiraterone (D4A) | This compound | 3β-hydroxysteroid dehydrogenase (3βHSD) | Potent antitumor activity prostatecancertopics.comaacrjournals.org |
| 5α-Abiraterone (3-keto-5α-abiraterone) | Δ4-Abiraterone | 5α-reductase | Androgenic; may promote cancer progression wikipedia.org |
| This compound Sulfate | This compound | SULT2A1 | Inactive drugbank.comnih.gov |
| N-oxide this compound Sulfate | This compound | CYP3A4, SULT2A1 | Inactive drugbank.comnih.gov |
The biotransformation of this compound into its various metabolites is a multi-step process involving several key enzymes.
3β-hydroxysteroid dehydrogenase (3βHSD): This enzyme is responsible for the initial conversion of this compound to Δ4-abiraterone (D4A). prostatecancertopics.comresearchgate.net This conversion is significant as D4A exhibits more potent anti-cancer properties than the parent drug. prostatecancertopics.com
5α-reductase: This enzyme acts on D4A, converting it to 5α-reduced metabolites, including the androgenic 3-keto-5α-abiraterone. wikipedia.orgoaepublish.com The irreversible nature of this steroid 5α-reduction means that once formed, these 5α-reduced metabolites are not converted back to this compound or D4A. prostatecancertopics.com
Other Enzymes: Cytochrome P450 enzymes, particularly CYP3A4, and sulfotransferase enzymes like SULT2A1 are crucial for the formation of the major inactive circulating metabolites, this compound sulfate and N-oxide this compound sulfate. drugbank.comnih.gov
Identification of Key this compound Metabolites (e.g., Δ4-Abiraterone, 5α-Abiraterone, this compound Sulfate, N-oxide this compound Sulfate)
Influence of Physiologic Factors on this compound Pharmacokinetics
The pharmacokinetics of this compound can be influenced by various physiologic factors. One area of research has been the effect of long-term administration. A study on the long-term pharmacokinetics of this compound acetate found that individual drug concentrations remained relatively constant over a period of up to 120 days, suggesting no significant changes with repeated administration. mdpi.comnih.gov This study also noted a positive correlation between patient age and this compound concentration, with older patients showing higher drug levels. mdpi.comnih.gov Long-term exposure to this compound and its metabolite D4A has been shown to lead to an increase in the expression and enzymatic activity of 5α-reductase, which could enhance the conversion of D4A to its androgenic 5α-reduced metabolites. nih.gov
Preclinical Investigations and Translational Research of Abiraterone
In Vitro Models for Abiraterone Research (e.g., Cell Lines)
In vitro studies utilizing diverse cancer cell lines have provided foundational insights into this compound's direct cellular effects and its mechanism of action. These models have been instrumental in characterizing this compound's cytotoxic and cytostatic properties, as well as exploring its impact on specific signaling pathways.
Adrenocortical Carcinoma (ACC) Cell Lines: this compound acetate (AA) has shown concentration-dependent effects on ACC cell lines. In the NCI-H295R cell line, AA treatment led to a reduction in cell viability, with reported decreases in cell number of 22% after two days of treatment with 200 nM AA, increasing to 47% after four days and 62% after six days oup.com. In contrast, the SW13 ACC cell line exhibited no significant change in viability when exposed to this compound oup.com. These findings suggest that the antiproliferative effects of this compound in ACC may be cell-line specific and potentially linked to specific molecular characteristics, such as the expression of the progesterone receptor (PgR), which has been implicated in mediating this compound's cytotoxic effects oup.comnih.gov.
Prostate Cancer Cell Lines: this compound has been evaluated in various prostate cancer cell lines, including androgen-sensitive (LNCaP) and androgen-insensitive (PC-3) models. Studies have shown that this compound and its acetate prodrug can reduce the viability of these cell lines compared to control treatments nih.gov. Specifically, in the 22Rv1 cell line, a prostate cancer model expressing PSMA, the CC50 (concentration causing 50% inhibition of cell growth) for an this compound conjugate (PSMA-Abi) was reported as 8.2 ± 3.1 μM, which was notably lower than that of this compound acetate (AbiAc) at 22.7 ± 3.3 μM, indicating enhanced cytotoxic potential for the conjugate in this model mdpi.com.
Breast Cancer Cell Lines: In the context of breast cancer, this compound has been investigated in models of triple-negative breast cancer (TNBC) that express the androgen receptor (AR), such as the MDA-MB-453 cell line aacrjournals.org. Furthermore, studies have explored this compound's activity in estrogen receptor-positive (ER+) breast cancer cell lines, including MCF7, HCC1428, and SUM44. These investigations suggest that this compound's role in ER+ breast cancer may be context-dependent, potentially influenced by prior hormone therapy and the presence of ESR1 mutations mdpi.com.
Other Cancer Cell Lines: this compound acetate has also been examined in colon cancer cell lines, where it demonstrated an inhibitory effect on tumor growth in vitro, with susceptibility noted in 59% of tumor cells at 50 μL and up to 67% at 300 μL researchgate.net.
Table 1: this compound Efficacy in Cancer Cell Lines
| Cell Line | Cancer Type | This compound Effect | Metric/Value | Reference |
| NCI-H295R | Adrenocortical Carcinoma | Reduced viability/cell number | 22% reduction at 2 days (200nM); 47% at 4 days; 62% at 6 days | oup.com |
| SW13 | Adrenocortical Carcinoma | No effect on viability | N/A | oup.com |
| 22Rv1 | Prostate Cancer | Cytotoxic | CC50 = 8.2 ± 3.1 μM | mdpi.com |
| PC-3 | Prostate Cancer | Cytotoxic | Comparable to AbiAc | mdpi.com |
| LNCaP | Prostate Cancer | Reduced viability | Compared to DMSO controls | nih.gov |
| PC3 | Prostate Cancer | Reduced viability | Compared to DMSO controls | nih.gov |
| MDA-MB-453 | Triple-Negative Breast Cancer | Used as model for AR+ TNBC | N/A | aacrjournals.org |
| MCF7 | Estrogen Receptor-positive Breast Cancer | Context-dependent role, influenced by prior therapy/ESR1 mutations | N/A | mdpi.com |
In Vivo Models for this compound Research (e.g., Xenograft Models, Patient-Derived Xenografts)
Preclinical in vivo studies, employing xenograft and patient-derived xenograft (PDX) models, have further validated this compound's anti-tumorigenic potential. These models allow for the assessment of this compound's efficacy in a more complex biological environment, including its impact on tumor growth, progression, and survival.
Adrenocortical Carcinoma (ACC) Xenografts: In immunodeficient mice bearing NCI-H295R cell line xenografts, this compound acetate (AA) demonstrated an ability to inhibit tumor growth. Treatment with AA led to a tumor volume inhibition (TVI) of 38% at day 36 post-treatment initiation (P = 0.07) and maintained a TVI of 34% by day 61 (P = 0.009) oup.com.
Prostate Cancer Xenografts and PDXs: this compound has been extensively studied in various prostate cancer xenograft and PDX models. In 22Rv1 xenografts, this compound acetate (AbiAc) achieved a 78% tumor growth inhibition (TGI), while a PSMA-targeted this compound conjugate (PSMA-Abi) showed a TGI of 65% mdpi.com. Studies utilizing castration-resistant prostate cancer (CRPC) patient-derived xenografts (PDXs) have revealed heterogeneity in response to AA. For instance, LuCaP 136CR PDXs exhibited an "ultraresponsive" phenotype with significant tumor progression inhibition and improved median survival (21.6 weeks vs. 6.8 weeks, P<0.0001), whereas LuCaP 35CR PDXs showed minimal tumor inhibition and no survival benefit aacrjournals.orgresearchgate.net. LuCaP 77CR and LuCaP 96CR PDXs demonstrated intermediate responses with modest tumor inhibition and survival benefits aacrjournals.orgresearchgate.net. In the LAPC-4 xenograft model, this compound was found to be less efficacious compared to a novel analog nih.gov. Furthermore, combination studies in CWR22Rv1 xenografts showed that this compound acetate, when combined with niclosamide, synergistically inhibited tumor size and weight compared to this compound alone oncotarget.com. In triple-negative breast cancer (TNBC) models, orthotopic xenograft experiments confirmed the efficacy of this compound acetate in combination with a Chk1 inhibitor aacrjournals.org.
Table 2: this compound Efficacy in Xenograft and PDX Models
| Model | Cancer Type | Treatment | Metric/Value | Notes | Reference |
| NCI-H295R Xenograft | Adrenocortical Carcinoma | This compound Acetate (AA) | Tumor Volume Inhibition (TVI) | 38% at day 36 (P=0.07); 34% at day 61 (P=0.009) | oup.com |
| 22Rv1 Xenograft | Prostate Cancer | AbiAc (reference) | Tumor Growth Inhibition (TGI) | 78% | mdpi.com |
| 22Rv1 Xenograft | Prostate Cancer | PSMA-Abi conjugate | Tumor Growth Inhibition (TGI) | 65% | mdpi.com |
| LuCaP 96CR PDX | Prostate Cancer (CRPC) | AA | Median Survival | 10 weeks (vs 5.75 weeks control, P=0.25) | aacrjournals.orgresearchgate.net |
| LuCaP 77CR PDX | Prostate Cancer (CRPC) | AA | Median Survival | 9.5 weeks (vs 7 weeks control, P=0.022) | aacrjournals.orgresearchgate.net |
| LuCaP 136CR PDX | Prostate Cancer (CRPC) | AA | Median Survival | 21.6 weeks (vs 6.8 weeks control, P<0.0001) | aacrjournals.orgresearchgate.net |
| LuCaP 35CR PDX | Prostate Cancer (CRPC) | AA | Median Survival | No significant benefit (vs control) | aacrjournals.orgresearchgate.net |
| CWR22Rv1 Xenograft | Prostate Cancer | This compound Acetate + Niclosamide | Synergistic tumor size/weight inhibition | Compared to this compound alone | oncotarget.com |
| Orthotopic Xenografts | Triple-Negative Breast Cancer (AR+) | This compound Acetate + Chk1 Inhibitor | Efficacy confirmed | In vivo efficacy | aacrjournals.org |
This compound Efficacy in Diverse Cancer Models (e.g., Adrenocortical Carcinoma, Breast Cancer, Ovarian Cancer)
The translational potential of this compound has been explored in various cancer types beyond prostate cancer, leveraging its mechanism of inhibiting androgen biosynthesis, a pathway relevant in several hormone-sensitive malignancies.
Adrenocortical Carcinoma (ACC): Preclinical investigations have established this compound's efficacy in ACC models. In vitro, this compound acetate reduced cell viability and hormone secretion in ACC cell lines and primary cultures, with its cytotoxic effect potentially mediated by progesterone receptor activity oup.comnih.govresearchgate.net. In vivo, this compound demonstrated anti-tumor activity in NCI-H295R xenografts, inhibiting tumor growth oup.comnih.govresearchgate.net.
Ovarian Cancer: The androgen receptor (AR) is expressed in a significant proportion of epithelial ovarian cancer (EOC) cases, providing a rationale for exploring AR-targeted therapies. While preclinical studies using enzalutamide, another AR inhibitor, showed reduced EOC xenograft growth, clinical trials with this compound acetate in recurrent EOC (CORAL trial) yielded rare responses. However, a subset of patients did achieve sustained clinical benefit, indicating that AR-targeting strategies may warrant further investigation in specific ovarian cancer subtypes nih.govtargetedonc.comresearchgate.net.
Table 3: this compound Efficacy in Different Cancer Types
| Cancer Type | Model Type | Observed Efficacy/Effect | Details | Reference |
| Adrenocortical Carcinoma | In vitro (NCI-H295R, primary cultures) | Reduced cell viability, inhibited hormone secretion | Concentration-dependent effect; requires PgR | oup.comnih.govresearchgate.net |
| Adrenocortical Carcinoma | In vivo (NCI-H295R xenografts) | Inhibited tumor growth | TVI of 34-38% | oup.comnih.govresearchgate.net |
| Prostate Cancer | In vivo (various xenografts/PDXs) | Inhibited tumor progression, improved survival | Heterogeneous responses observed in PDXs | nih.govmdpi.comaacrjournals.orgresearchgate.netnih.govoncotarget.comdovepress.comicr.ac.ukaacrjournals.orgnih.gov |
| Triple-Negative Breast Cancer (AR+) | In vitro (MDA-MB-453) | Androgen-dependent growth model | Used in combination studies | aacrjournals.org |
| Triple-Negative Breast Cancer (AR+) | In vivo (orthotopic xenografts) | Combination with Chk1 inhibitor showed efficacy | Confirmed in vivo efficacy | aacrjournals.org |
| Estrogen Receptor-positive Breast Cancer | In vitro/Clinical | Limited benefit observed | Context-dependent role suggested | mdpi.commdpi.comnih.gov |
| Epithelial Ovarian Cancer | In vivo (preclinical models) | Reduced xenograft growth (Enzalutamide) | Supports exploration of AR inhibitors | nih.gov |
| Epithelial Ovarian Cancer | Clinical (CORAL Trial) | Rare responses, sustained clinical benefit in a subset | ORR 2.4%, CBR 26% | nih.govtargetedonc.comresearchgate.net |
Compound List:
this compound
this compound acetate (AA)
PSMA-Abi
AbiAc
Enzalutamide
Bicalutamide
Seviteronel
Mitotane
Cabazitaxel
Niclosamide
Onvansertib
Galeterone
D4A (Δ4-abiraterone)
CB7598
Enobosarm
Prednisone
Q & A
Q. How to resolve discrepancies between preclinical efficacy and clinical trial outcomes for this compound-based therapies?
- Answer :
- Tumor heterogeneity : Use multi-region sequencing in PDX models to identify resistant subclones.
- Dosing : Compare preclinical doses (mg/kg) to human equivalent doses (HED) using FDA guidelines.
- Biomarker discordance : Validate surrogate endpoints (e.g., PSA) with tissue-based AR-V7 expression .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


