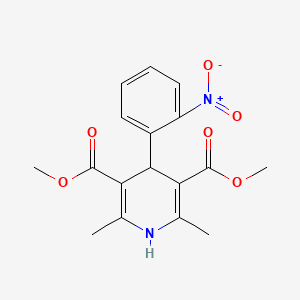
Nifedipine
Description
This compound is a dihydropyridine, a methyl ester and a C-nitro compound. It has a role as a calcium channel blocker, a vasodilator agent, a tocolytic agent and a human metabolite.
This compound, or BAY a 1040, is a first generation dihydropyridine L-type calcium channel blocker, similar to [nicardipine]. This compound was developed by Bayer and first described in the literature, along with other dihydropyridines, in 1972. Since this compound's development, second and third generation dihydropyridines have been developed with slower onsets and longer durations of action. The most popular of the third generation dihydropyridines is [amlodipine]. this compound was granted FDA approval on 31 December 1981.
This compound is a Dihydropyridine Calcium Channel Blocker. The mechanism of action of this compound is as a Calcium Channel Antagonist.
This compound is a first generation calcium channel blocker used to treat hypertension and angina pectoris. This compound therapy is associated with a low rate of serum enzyme elevations and has been linked to several instances of clinically apparent acute liver injury.
This compound is a natural product found in Homo sapiens with data available.
This compound is a dihydropyridine calcium channel blocking agent. This compound inhibits the transmembrane influx of extracellular calcium ions into myocardial and vascular smooth muscle cells, causing dilatation of the main coronary and systemic arteries and decreasing myocardial contractility. This agent also inhibits the drug efflux pump P-glycoprotein which is overexpressed in some multi-drug resistant tumors and may improve the efficacy of some antineoplastic agents. (NCI04)
This compound can cause developmental toxicity, female reproductive toxicity and male reproductive toxicity according to state or federal government labeling requirements.
A potent vasodilator agent with calcium antagonistic action. It is a useful anti-anginal agent that also lowers blood pressure.
Properties
IUPAC Name |
dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HYIMSNHJOBLJNT-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C(C(=C(N1)C)C(=O)OC)C2=CC=CC=C2[N+](=O)[O-])C(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H18N2O6 | |
| Record name | NIFEDIPINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20738 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | nifedipine | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Nifedipine | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
60299-11-8 (mono-hydrochloride) | |
| Record name | Nifedipine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021829254 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2025715 | |
| Record name | Nifedipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2025715 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
346.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Nifedipine appears as odorless yellow crystals or powder. Tasteless. (NTP, 1992), Solid | |
| Record name | NIFEDIPINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20738 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Nifedipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015247 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
less than 1 mg/mL at 67.1 °F (NTP, 1992), Insoluble, Solubility at 20 °C (g/L): acetone 250, methylene chloride 160, chloroform 140, ethyl acetate 50, methanol 26, ethanol 17, In water, 1.7X10-5 mol/L = 5.9 mg/L at 25 °C, 1.77e-02 g/L | |
| Record name | NIFEDIPINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20738 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Nifedipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01115 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nifedipine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7775 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Nifedipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015247 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
2.6X10-8 mm Hg at 25 °C | |
| Record name | Nifedipine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7775 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
Dimethyl 2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate, Dimethyl 2,6-dimethyl-4-(2_nitrosophenyl)pyridine-3,5-dicarboxylate, Methyl 2-(2-nitrobenzylidene)-3-oxobutanoate, Methyl 3-aminobut-2-enoate | |
| Record name | Nifedipine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7775 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Yellow crystals | |
CAS No. |
21829-25-4, 193689-82-6, 915092-63-6 | |
| Record name | NIFEDIPINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20738 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, dimethyl ester, radical ion(1-) | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=193689-82-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nifedipine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=21829-25-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, 3,5-dimethyl ester, radical ion(1+) | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=915092-63-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nifedipine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021829254 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nifedipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01115 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | nifedipine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757242 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nifedipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2025715 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Nifedipine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.040.529 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NIFEDIPINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/I9ZF7L6G2L | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Nifedipine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7775 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Nifedipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015247 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
342 to 345 °F (NTP, 1992), 172-174 °C, 172 - 174 °C | |
| Record name | NIFEDIPINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20738 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Nifedipine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01115 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nifedipine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7775 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Nifedipine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015247 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Nifedipine Pharmacology: Advanced Mechanistic Investigations
Molecular Mechanisms of Voltage-Gated L-Type Calcium Channel Inhibition by Nifedipine
This compound exerts its primary pharmacological effects by blocking voltage-gated L-type calcium channels (LTCCs) drugbank.comnih.govontosight.ai. These channels are crucial for calcium entry into various excitable cells, including vascular smooth muscle and myocardial cells drugbank.comnih.govontosight.aiconsensus.app. By inhibiting this influx, this compound reduces intracellular calcium concentrations, leading to relaxation of vascular smooth muscle and decreased myocardial contractility nih.govontosight.ai.
Allosteric Modulation of CaV1.2 and CaV1.3 Subunits
L-type calcium channels are composed of several subunits, with the alpha-1 (α1) subunit being the primary pore-forming component that also contains the drug binding sites sigmaaldrich.com. The CaV1.2 and CaV1.3 subtypes are prominent members of the LTCC family sigmaaldrich.comnih.gov. Research indicates that this compound modulates CaV1.2 and CaV1.3 channels, showing a more potent effect on CaV1.2 compared to CaV1.3 nih.govnih.gov. Studies investigating the molecular determinants of this differential modulation have highlighted the role of specific amino acid residues in the transmembrane domains and extracellular loops of these subunits nih.govnih.gov. For instance, variations in the IIIS5 transmembrane domain and the extracellular IIIS5-3P loop region contribute significantly to the difference in this compound potency between CaV1.2 and CaV1.3 channels nih.govnih.gov.
Data from research indicates the following IC50 values for this compound block on different CaV subtypes:
| CaV Subtype | IC50 (nM) | Reference |
| CaV1.2 | 22 ± 2 | nih.gov |
| CaV1.3 | 289 ± 30 | nih.gov |
Mutagenesis studies have further elucidated the impact of specific residue substitutions on this compound's potency. For example, inserting the extracellular IIIS5-3P loop from CaV1.2 into CaV1.3 or substituting a specific serine residue (S1100) in CaV1.3 to an alanine residue (as found in CaV1.2) reduced the IC50 of this compound for CaV1.3, making it more sensitive to the drug nih.govnih.gov. Combining certain mutations can further enhance this compound's potency on CaV1.3 nih.govnih.gov.
Interaction with Alpha-1 Subunits of Calcium Channels
This compound primarily interacts with the alpha-1 (α1) subunit of L-type calcium channels ontosight.aisigmaaldrich.com. This subunit is the main functional component responsible for ion permeation and gating sigmaaldrich.com. The binding of this compound to the α1 subunit inhibits the influx of calcium ions into the cell ontosight.ai. While the α1 subunit contains the primary drug binding sites, auxiliary subunits, such as the beta (β) subunit, can also influence drug sensitivity and efficacy sigmaaldrich.comconsensus.app. This compound has been identified as an inhibitor of several alpha-1 subunits, including alpha-1C (CaV1.2), alpha-1D (CaV1.3), and alpha-1S (CaV1.1) drugbank.com.
Differential Potency on L-Type vs. T-Type Calcium Channels
This compound is known to be significantly more potent in blocking L-type calcium channels compared to T-type calcium channels frontiersin.org. While L-type channels are high-voltage activated channels primarily involved in muscle contraction and hormone release, T-type channels are low-voltage activated and play roles in pacemaker activity and neuronal firing sigmaaldrich.come-jcpp.orgresearchgate.net. Studies have shown that this compound inhibits L-type channels at much lower concentrations than T-type channels frontiersin.org. However, this compound is capable of blocking T-type calcium channels, including CaV3.1, CaV3.2, and CaV3.3, albeit with lower potency nih.gov. The potency varies among T-type subtypes, with CaV3.2 appearing to be the most sensitive to this compound among the T-type channels studied nih.gov.
Research findings on this compound's potency on T-type channels:
| T-Type Subtype | IC50 (µM) | Maximum Block (%) | Cellular System | Reference |
| CaV3.1 | 109 | 23 | Xenopus oocytes | nih.gov |
| CaV3.2 | 5 | 41 | Xenopus oocytes | nih.gov |
| CaV3.3 | 243 | 47 | Xenopus oocytes | nih.gov |
| Endogenous LVA, fast | 22 | 81 | Thalamic neurons | nih.gov |
| Endogenous LVA, slow | 28 | 51 | Thalamic neurons | nih.gov |
Impact on Intracellular Calcium Dynamics and Signaling Pathways
The primary consequence of L-type calcium channel blockade by this compound is the reduction of intracellular calcium influx nih.govontosight.ai. This decrease in intracellular calcium ([Ca2+]i) is the basis for its vasodilatory and negative inotropic effects nih.govontosight.ai. However, some studies suggest that this compound might also influence intracellular calcium dynamics beyond simple channel blockade. Research in cultured human smooth muscle cells indicated that this compound could induce an increase in [Ca2+]i under certain conditions, an effect potentially linked to the activation of store-operated channels and modulated by tyrosine kinase activity and the sarcoplasmic reticulum Ca2+-ATPase pump nih.gov. Furthermore, studies in embryonic stem cells suggest that LTCCs and the proper maintenance of intracellular calcium concentration and pathways are essential for cardiac gene expression, differentiation, and function, and this compound treatment at early stages can inhibit cardiac mesoderm formation and lineage commitment plos.org.
Cellular and Subcellular Effects of this compound Beyond Calcium Channel Blockade
Modulation of Nitric Oxide Production and Endothelial Function
This compound has been reported to modulate nitric oxide (NO) production and improve endothelial function imrpress.comtandfonline.comfrontiersin.orgmdpi.com. NO is a crucial signaling molecule involved in vasodilation and maintaining vascular health tandfonline.comnih.govguidetopharmacology.org. Studies have demonstrated that this compound can increase endothelial NO bioavailability imrpress.comfrontiersin.org. This effect may be mediated, at least in part, by antioxidative mechanisms, as this compound has been shown to reduce the formation of reactive oxygen species in endothelial cells imrpress.comtandfonline.comahajournals.org. Increased NO levels or bioavailability contribute to improved endothelial function and can help counteract endothelial dysfunction imrpress.comtandfonline.commdpi.com. Research in patients with coronary heart disease and essential hypertension has shown that this compound treatment can improve endothelial function and reduce markers of oxidative stress imrpress.commdpi.com.
Furthermore, some studies suggest that this compound's antiaggregatory properties might be mediated via a nitric oxide-dependent process tandfonline.com. This compound has also been shown to stimulate Ca2+ and NO formation in endothelial cells through a kinase-dependent mechanism tandfonline.com.
Beyond its effects on calcium channels and NO, this compound has been investigated for other potential effects, such as modulating immune responses, inhibiting tumor growth, and influencing the metabolism of chondrocytes and mesenchymal stem cells frontiersin.orgfrontiersin.org. Some research also suggests a previously uncharacterized action of this compound on central synaptic transmission, facilitating neurotransmitter release independently of calcium channels, which might explain some neurological side effects pnas.orgnih.gov.
Influence on Mitochondrial Respiration and Bioenergetics in Specific Cell Types
Studies have explored the impact of this compound on cellular energy metabolism, particularly mitochondrial respiration and ATP production, in various cell types. In human chondrocytes and bone marrow-derived mesenchymal stem cells (BMMSCs), this compound has been shown to downregulate mitochondrial respiration and ATP production researchgate.netnih.gov. Electron microscopy analysis of cartilage explants suggested that a portion of mitochondria lose activity in response to this compound treatment researchgate.netnih.gov. While mitochondrial respiration and ATP production were inhibited in both cell types, a switch towards glycolytic metabolism was observed only in chondrocytes researchgate.netnih.gov. This suggests a cell-type specific metabolic adaptation to this compound's effects on mitochondrial function nih.gov.
The mechanism underlying this compound's influence on mitochondrial function may involve increased nitric oxide (NO) accumulation researchgate.netnih.gov. NO is known to inhibit mitochondrial activity, and this compound has been shown to stimulate NO activity in BMMSCs and particularly in chondrocytes researchgate.netnih.govahajournals.org. This suggests that NO may partially mediate the metabolic effects of this compound in these cells researchgate.netnih.gov.
Furthermore, in hypoxic A549 cells, this compound has been shown to reduce mitochondrial calcium overload and subsequent reactive oxygen species (ROS) generation nih.gov. This protective effect is attributed to the reduction in cytosolic calcium levels mediated by this compound's L-type calcium channel blocking activity nih.gov.
Data illustrating the effects of this compound on mitochondrial respiration parameters in chondrocytes and BMMSCs are presented in the table below.
| Cell Type | Parameter | This compound Effect (vs Control) | Source |
| Chondrocytes | Mitochondrial Respiration | Downregulated | researchgate.netnih.gov |
| Chondrocytes | ATP Production | Downregulated | researchgate.netnih.gov |
| Chondrocytes | Glycolytic Capacity | Enhanced | nih.gov |
| Chondrocytes | Spare Respiratory Capacity | Reduced (instant treatment) | nih.gov |
| BMMSCs | Mitochondrial Respiration | Downregulated | researchgate.netnih.gov |
| BMMSCs | ATP Production | Downregulated | researchgate.netnih.gov |
| BMMSCs | Glycolytic Switch | Not Observed | nih.gov |
Effects on Neurotransmitter Release Mechanisms Independent of Canonical Calcium Channels
Beyond its well-established effects on L-type calcium channels, this compound has been found to influence neurotransmitter release through mechanisms independent of these canonical channels nih.govnih.govresearchgate.netpnas.org. Research in central synapses has demonstrated that this compound can cause a long-lasting facilitation of tetrodotoxin-insensitive spontaneous glutamate release nih.govnih.govpnas.org. This effect is notable because it is independent of its L-type calcium channel blocking activity and is not replicated by other dihydropyridines like nimodipine or nicardipine nih.govnih.govpnas.org.
The facilitation of spontaneous glutamate release by this compound appears to be largely calcium-independent, as it was not inhibited by agents that interfere with calcium, such as Cd2+, thapsigargin, or BAPTA-AM nih.govnih.govpnas.org. This suggests that this compound may act on the neurotransmitter release process downstream of calcium entry or release nih.govnih.govpnas.org. Furthermore, this effect does not appear to be mediated by protein kinases A or C nih.govnih.gov.
The concentration-dependent effect of this compound on spontaneous glutamate release has been reported, with an EC50 of 7.8 μM and effects observed at concentrations as low as 100 nM nih.govnih.govpnas.org. At a concentration of 10 μM, this compound has been shown to increase spontaneous glutamate release by approximately 14.7-fold nih.govnih.gov.
This calcium-independent facilitation of neurotransmitter release by this compound represents a previously uncharacterized action that could contribute to some of its observed side effects nih.govnih.govpnas.org. While L-type calcium channels are typically located on cell bodies and dendrites, and N- and P/Q-type calcium channels are primarily linked to presynaptic neurotransmitter release, this compound's effect on spontaneous release suggests a more direct interaction with the release machinery itself or the membrane fusion process pnas.orgpnas.orgphysiology.org.
Nifedipine Pharmacodynamics: Comprehensive Research Perspectives
Vascular Smooth Muscle Relaxation and Peripheral Arterial Vasodilation Mechanisms
The primary mechanism by which nifedipine induces vascular smooth muscle relaxation and peripheral arterial vasodilation is the inhibition of the transmembrane influx of calcium ions. This compound specifically targets voltage-gated L-type calcium channels located in the cell membranes of vascular smooth muscle cells. drugbank.comnih.govbauschhealth.compfizermedicalinformation.compatsnap.combauschhealth.compatsnap.com
Under normal physiological conditions, the contraction of vascular smooth muscle is dependent on the movement of extracellular calcium ions into the cells through these channels. bauschhealth.compfizermedicalinformation.combauschhealth.com This influx of calcium is a critical step in initiating and maintaining the contractile process. By blocking the L-type calcium channels, this compound prevents calcium from entering the smooth muscle cells during depolarization. drugbank.comnih.govbauschhealth.compfizermedicalinformation.combauschhealth.com The resulting reduction in intracellular calcium concentration leads to the relaxation of the vascular smooth muscle. patsnap.com
This relaxation causes peripheral arterial vasodilation, which in turn reduces peripheral vascular resistance. drugbank.combauschhealth.compfizermedicalinformation.combauschhealth.compatsnap.comdroracle.airesearchgate.net The decrease in peripheral vascular resistance is a key factor in the antihypertensive effect of this compound, as increased peripheral vascular resistance is an underlying cause of hypertension. bauschhealth.compfizermedicalinformation.combauschhealth.comresearchgate.net Studies have demonstrated that the increase in active tension in vascular smooth muscle, contributing to elevated peripheral resistance, reflects an increase in cytosolic free calcium. bauschhealth.compfizermedicalinformation.combauschhealth.comresearchgate.net this compound's action directly counteracts this by limiting calcium influx. bauschhealth.compfizermedicalinformation.combauschhealth.comresearchgate.net
Beyond its direct calcium channel blocking effects, research also suggests that this compound may influence endothelium-derived factors. Some studies indicate that this compound, in addition to its calcium antagonistic properties in vascular smooth muscle cells, can stimulate the release of nitric oxide (NO) in the endothelium and also help preserve NO concentration. ahajournals.org NO is a potent vasodilator and an endothelium-derived relaxing factor. ahajournals.org This potential dual mode of action, involving both inhibition of smooth muscle L-type calcium channel influx and influencing endothelial NO, may contribute to its vasorelaxing effect and the preservation of endothelial function. ahajournals.org However, there is conflicting evidence regarding the extent to which dihydropyridines like this compound alter NO synthesis or release, with some earlier studies suggesting inhibition of NO release from certain endothelial cells and arteries. ahajournals.org
Coronary Artery Dilation and Myocardial Oxygen Supply Augmentation
This compound is also known to dilate coronary arteries, which contributes to its effectiveness in treating angina pectoris. drugbank.comnih.govpfizermedicalinformation.compatsnap.compatsnap.commims.com This dilation affects both the main coronary arteries and coronary arterioles, in both normal and ischemic regions of the myocardium. pfizermedicalinformation.comhres.cajournals.co.za
The mechanism behind coronary artery dilation is similar to that in peripheral arteries: the blockade of L-type calcium channels in the smooth muscle of the coronary vessels. drugbank.comnih.gov By reducing calcium influx, this compound causes relaxation of the coronary vascular smooth muscle, leading to vasodilation. drugbank.comnih.govmims.com
A significant effect of this compound is its potent inhibition of coronary artery spasm. pfizermedicalinformation.comhres.cajournals.co.za This property is particularly important in vasospastic angina (Prinzmetal's or variant angina), where transient spasms of the coronary arteries restrict blood flow to the heart muscle. pfizermedicalinformation.comhres.cajournals.co.za By preventing or relieving these spasms, this compound increases myocardial oxygen delivery. pfizermedicalinformation.comhres.ca
In the context of chronic stable angina, the dilation of coronary arteries and reduction in peripheral vascular resistance (afterload) collectively reduce the workload of the heart. pfizermedicalinformation.compatsnap.comhres.cajournals.co.za This reduction in afterload decreases myocardial energy consumption and oxygen requirements. pfizermedicalinformation.comhres.cajournals.co.za While dilation of coronary arteries can increase oxygen supply, the reduction in oxygen demand due to decreased afterload is considered a primary mechanism for its effectiveness in chronic stable angina. pfizermedicalinformation.comhres.cajournals.co.za Studies have shown that this compound can improve manifestations of myocardial ischemia during exercise without altering the rate-pressure product (a measure of oxygen utilization), suggesting a mechanism related to enhanced blood flow to ischemic areas or favorable alteration of oxygen demand determinants. nih.gov
Research has also explored the effects of this compound on myocardial energetics. Studies in patients with congestive heart failure have shown that this compound can enhance myocardial performance while increasing coronary blood flow and favorably altering the myocardial oxygen supply-demand balance. ahajournals.org This involves an increase in cardiac index and a decrease in systemic vascular resistance, along with diminished coronary vascular resistance and an increase in coronary sinus oxygen saturation. ahajournals.org
Hemodynamic Responses and Cardiorenal Protective Effects Research
This compound elicits significant hemodynamic responses primarily through its potent vasodilatory action. The reduction in peripheral arterial resistance leads to a decrease in systemic blood pressure. drugbank.comnih.govbauschhealth.compfizermedicalinformation.combauschhealth.compatsnap.comdroracle.airesearchgate.net This effect is often more pronounced with formulations that result in rapid increases in plasma concentration. ecrjournal.comnih.gov
Studies comparing the hemodynamic effects of this compound with other vasodilators like nitroprusside in conditions such as severe chronic congestive heart failure have provided insights into its specific profile. While both drugs can produce similar reductions in systemic vascular resistance, this compound has been shown to cause a larger decrease in mean blood pressure but a smaller increase in cardiac index compared to nitroprusside. nih.gov this compound also had a lesser effect on right and left ventricular filling pressure and pulmonary vascular resistance in these studies. nih.gov
The impact of this compound on renal hemodynamics has also been investigated. Research in anesthetized rats suggests that this compound can increase urine output and natriuresis. mdpi.com While it may not have major effects on basal mean arterial pressure and renal blood flow at lower doses, higher doses have shown some attenuation of neuropeptide Y-induced alterations in mean arterial pressure, renal blood flow, and renovascular resistance. mdpi.com This suggests potential effects on renal vasculature, although the mechanisms and clinical significance in humans require further investigation.
Research has also explored potential cardiorenal protective effects associated with this compound, particularly in the context of hypertension. Studies in spontaneously hypertensive rats have investigated the effects of this compound, alone or in combination with hydrochlorothiazide, on blood pressure variability, baroreflex sensitivity, and organ protection. researchgate.net These studies have assessed pathological changes in ventricles, kidneys, and aortae. researchgate.net While some studies in animal models suggest a reduction in end-organ damage with this compound treatment, the direct cardiorenal protective mechanisms beyond blood pressure reduction are areas of ongoing research. researchgate.net
Data on hemodynamic responses can be complex and vary depending on the study population, formulation, and presence of underlying conditions. The following table summarizes some observed hemodynamic changes from a study in patients with severe chronic congestive heart failure comparing this compound and nitroprusside:
| Hemodynamic Parameter | This compound (Change from Baseline) | Nitroprusside (Change from Baseline) | p-value |
| Systemic Vascular Resistance (SVR) | -29% ± 13% | -29% ± 12% | NS |
| Cardiac Index | +20% ± 20% | +40% ± 24% | < 0.02 |
| Mean Blood Pressure | -16% ± 9% | -8% ± 10% | < 0.05 |
| Mean Pulmonary Artery Wedge Pressure | -13% ± 24% | -36% ± 21% | < 0.001 |
| Pulmonary Vascular Resistance | -6% ± 42% | -26% ± 46% | NS |
| Mean Right Atrial Pressure | 0% | Decrease | < 0.05 |
| Left Ventricular Stroke Work Index | No Change | Increase | < 0.05 |
*Data derived from a study comparing this compound and nitroprusside in patients with severe chronic congestive heart failure. nih.gov NS = Not Significant.
Impact on Sympathetic Nervous System Activity and Baroreflex Sensitivity
The vasodilatory effects of this compound can trigger reflex activation of the sympathetic nervous system. nih.govecrjournal.comnih.govjacc.orgahajournals.orgahajournals.org This occurs as a compensatory response to the reduction in blood pressure. The sympathetic nervous system activation can lead to an increase in heart rate and plasma norepinephrine levels. ecrjournal.comnih.govjacc.orgahajournals.orgahajournals.org
Research using techniques like microneurography has investigated the influence of this compound on muscle sympathetic nerve activity (MSNA) and skin sympathetic nerve activity (SSA). Studies in healthy volunteers have shown that both short-acting and slow-release formulations of this compound can markedly activate MSNA and increase plasma norepinephrine. nih.govjacc.org However, heart rate increase was primarily observed with short-acting this compound, not the slow-release formulation. nih.govjacc.org this compound appeared to have no effect on SSA in these studies. nih.govjacc.org This suggests that this compound may activate cardiac and peripheral sympathetic nerves differently depending on the pharmacokinetic profile. nih.govjacc.org
The rate at which blood pressure is lowered by this compound appears to influence the degree of sympathetic activation. ecrjournal.com Rapid reductions in blood pressure, typically seen with immediate-release formulations, are more likely to induce reflex tachycardia and increased plasma noradrenaline levels. nih.govecrjournal.com In contrast, formulations providing a slower, more controlled release of this compound may result in a more gradual decrease in blood pressure with less or no reflex sympathetic activation. ecrjournal.comjddtonline.info
The effect of this compound on baroreflex sensitivity has also been a subject of research. The baroreflex is a crucial mechanism for regulating blood pressure and heart rate. Some studies suggest that this compound may potentiate baroreflex control. oup.comnih.govcapes.gov.br Research using microneurography in healthy humans has provided direct evidence that this compound can augment cardiopulmonary baroreflex control of sympathetic nerve activity. nih.gov This was demonstrated by an augmented percentage increase in MSNA during selective unloading of cardiopulmonary baroreceptors after this compound administration. nih.gov this compound has also been shown to augment arterial baroreceptor modulation of heart rate during dynamic increases in arterial pressure. capes.gov.br
However, other studies on the effect of calcium channel blockers on baroreflex sensitivity have yielded conflicting results. oup.comnih.gov Some research in rats indicated that while verapamil depressed baroreflex sensitivity, this compound did not show a similar effect. nih.gov The complex interplay between this compound's direct vasodilatory effects and its influence on the baroreflex and sympathetic nervous system is an active area of investigation.
Dose-Dependent Pharmacodynamic Variability and Clinical Correlates
The pharmacodynamic effects of this compound can exhibit dose-dependent variability, and understanding these relationships is crucial for predicting clinical responses. Research has aimed to establish concentration-effect relationships for this compound, particularly regarding its antihypertensive effects. nih.govahajournals.orgnih.govahajournals.org
Studies in hypertensive subjects have demonstrated significant correlations between plasma this compound concentrations and the reduction in blood pressure. nih.govahajournals.orgnih.gov These relationships are often described as sigmoidal. nih.gov The magnitude of the blood pressure reduction by this compound has been shown to be directly proportional to the pretreatment blood pressure. ahajournals.orgahajournals.org
Research investigating different doses of this compound has shown dose-dependent reductions in blood pressure. For instance, a study using this compound tablets at doses of 20 mg, 40 mg, and 60 mg found that all three doses significantly lowered blood pressure, with the reduction being larger and lasting longer after the 60 mg dose compared to the 20 mg dose. nih.gov
The rate of increase in plasma this compound concentration is also a critical factor influencing the pharmacodynamic response. ecrjournal.comnih.gov Rapid increases in concentration, typically associated with immediate-release formulations, can lead to a more pronounced and rapid fall in blood pressure, often accompanied by reflex tachycardia. nih.govecrjournal.comnih.gov Formulations designed for slower release, such as the Gastrointestinal Therapeutic System (GITS) formulation, provide a smoother plasma concentration profile, resulting in a more gradual and sustained blood pressure reduction with less reflex sympathetic activation. ecrjournal.comnih.govjddtonline.info
Studies have explored the relationship between this compound plasma concentration and heart rate, often observing a significant positive correlation, particularly with formulations leading to rapid absorption. nih.gov However, the variability in this relationship can be considerable among individuals. nih.gov
Research continues to refine the understanding of this compound's dose-dependent effects and their relationship to plasma concentrations, aiming to optimize therapeutic strategies and predict individual patient responses. ahajournals.orgahajournals.orgnih.govmdpi.com
Adverse Event Mechanisms and Safety Research for Nifedipine
Mechanisms of Reflex Tachycardia and Sympathetic Activation with Short-Acting Formulations
Short-acting formulations of nifedipine are associated with a rapid onset of vasodilation and a consequent swift reduction in blood pressure. nih.govdroracle.airesearchgate.net This sudden decrease in blood pressure triggers the body's baroreceptor reflex, a compensatory mechanism aimed at restoring blood pressure homeostasis. droracle.ai The baroreceptor reflex involves the activation of the sympathetic nervous system. droracle.aineurology.org This sympathetic activation leads to an increase in heart rate (reflex tachycardia) and cardiac output, as well as increased plasma catecholamine and plasma renin activity. droracle.aineurology.org This reflex sympathetic activation is a key mechanism underlying adverse effects like palpitations and flushing observed with immediate-release this compound. nih.gov Studies have shown that rapid increases in plasma concentrations of this compound, such as those occurring with immediate-release capsules, are particularly prone to inducing baroreflex sympathetic activation. researchgate.net Conversely, modified-release formulations that provide a more gradual increase and sustained plasma level are less associated with this effect. researchgate.net
Dose-Related Risk of Increased Mortality and Myocardial Infarction in Coronary Artery Disease
Several meta-analyses and studies in the 1990s raised concerns regarding a potential dose-related increase in mortality and myocardial infarction risk with short-acting this compound in patients with coronary artery disease. nih.govahajournals.orgresearchgate.net While the precise mechanisms are still under investigation, several plausible explanations have been proposed, including proischemic, proarrhythmic, negative inotropic, and prohemorrhagic effects. nih.govahajournals.org A meta-analysis of 16 randomized secondary-prevention trials of this compound showed a statistically significant adverse effect on total mortality, with a notable dose-response relationship. nih.govahajournals.org
| Daily Dose of this compound (mg) | Risk Ratio for Total Mortality (95% CI) |
| 30 to 50 | 1.06 (0.89 to 1.27) |
| 60 | 1.18 (0.93 to 1.50) |
| 80 | 2.83 (1.35 to 5.93) |
Data derived from a meta-analysis of randomized secondary-prevention trials. nih.govahajournals.org
This data indicates a significantly increased risk of mortality at higher daily doses of short-acting this compound in patients with coronary artery disease. nih.govahajournals.org
Proischemic and Proarrhythmic Effects
Proischemic effects may result from reflex increases in sympathetic activity or a reduction of coronary perfusion pressure induced by short-acting calcium channel blockers. medcentral.com The rapid vasodilation and subsequent hypotension can lead to a reduction in coronary perfusion pressure, potentially aggravating pre-existing myocardial ischemia, particularly in patients with hypotension and/or tachycardia. medcentral.com Some studies have suggested a "coronary steal" phenomenon, where this compound might cause disproportionate dilation of coronary arteries adjacent to ischemic areas, diverting blood flow away from compromised regions, although the evidence supporting this in humans is considered speculative by some. medcentral.comahajournals.org
Short-acting this compound has also been suggested to have proarrhythmic effects, potentially contributing to an increased risk of stroke, especially in patients with atrial fibrillation. neurology.org Reflex sympathetic activation following an abrupt drop in blood pressure may be linked to ventricular arrhythmias. droracle.ai
Negative Inotropic and Prohemorrhagic Mechanisms
While this compound is a dihydropyridine and generally considered to have less pronounced negative inotropic effects compared to non-dihydropyridines like verapamil or diltiazem, it can still exert a negative inotropic effect, particularly at higher doses or in susceptible individuals. nih.govmedcentral.comahajournals.org This can lead to a reduction in myocardial contractility. nih.gov The drug's negative inotropic effect combined with potent hypotension may result in further myocardial ischemic damage. neurology.org
Prohemorrhagic effects have also been attributed to calcium antagonists, including this compound, potentially due to antiplatelet and vasodilatory actions. nih.govahajournals.org These effects may contribute to an increased risk of hemorrhagic stroke. neurology.org
Hepatotoxicity Research and Proposed Mechanisms
This compound has been associated with liver injury, although it is considered a rare adverse effect. nih.govresearchgate.net The severity of liver injury can range from mild, transient enzyme elevations to more severe forms like cholestatic hepatitis or an alcoholic hepatitis-like syndrome. nih.govresearchgate.net The mechanism of this compound-induced hepatotoxicity is not fully understood but is suspected to involve the production of a toxic or immunogenic intermediate during its metabolism in the liver, primarily via the CYP3A4 enzyme pathway. fda.govnih.gov While rare, clinically apparent hepatitis can occur and may take months to resolve after discontinuation of the drug. researchgate.net Liver biopsy in reported cases has shown features such as infiltration of immune cells, cholestasis, and steatohepatitis. researchgate.net this compound has also been listed as a drug that can cause macrovesicular steatosis. medscape.com
Neurological Adverse Events and Underlying Mechanisms (e.g., cerebral ischemia)
Neurological adverse events, including cerebral ischemia and stroke, have been associated with the use of short-acting this compound, particularly in elderly hypertensive patients. nih.govneurology.org Possible mechanisms for cerebral ischemia include transient severe systemic hypotension and a more effective vasodilating action of the drug on peripheral resistance vessels compared to cerebral vessels. neurology.org Hemodynamic fluctuations induced by the rapid and potent hypotensive effect of short-acting this compound can lead to reduced cerebral perfusion, increasing the risk of ischemic events. neurology.orgresearchgate.net Prohemorrhagic and proischemic effects, as discussed earlier, may also contribute to the increased stroke risk. neurology.org The use of immediate-release this compound for hypertensive crises has been particularly linked to severe hypotension, cerebral ischemia or infarction, and stroke. nih.govmedcentral.comresearchgate.net
Gingival Hyperplasia: Pathophysiological Basis
Gingival hyperplasia, an abnormal enlargement of the gums, is a known side effect of certain medications, including this compound. medscape.commdpi.comnih.gov The pathophysiology of drug-induced gingival overgrowth is multifactorial and not entirely understood, but it is believed to involve the interaction of the drug with gingival fibroblasts and keratinocytes. medscape.commdpi.com
One proposed mechanism involves the inhibition of calcium influx into gingival fibroblasts, which can interfere with collagen metabolism. medscape.com Calcium channel blockers like this compound may inhibit the degradation of collagen and extracellular matrix components, leading to their accumulation in the gingival tissue. medscape.com
Another hypothesis suggests that this compound may affect fibroblast proliferation and the cytokine network. medscape.com While some studies initially suggested increased keratinocyte proliferation, later research indicated that gingival hyperplasia in this compound-treated patients might be related to prolonged cell life rather than enhanced proliferation. mdpi.com
Drug Withdrawal Effects (e.g., rebound hypertension)
The discontinuation of antihypertensive medications, including calcium channel blockers like this compound, can potentially lead to changes in blood pressure and other symptoms. While some sources suggest that a "rebound" effect, characterized by a significant and sudden increase in blood pressure above pre-treatment levels, may not be a universal phenomenon with this compound, gradual withdrawal is generally recommended as sound clinical practice. drugs.comfda.gov Abrupt cessation of calcium channel blockers can potentially lead to rebound hypertension, increased angina, or worsening of arrhythmias as the body has adapted to the medication's effects. droracle.ai
Studies investigating the effects of this compound withdrawal have yielded varying results depending on the patient population and study design. In a study of patients with stable angina pectoris, abrupt cessation of this compound (20 mg four times daily for 5 weeks) resulted in a rebound decrease in exercise tolerance and an increase in exercise-induced myocardial ischemia on the first day of withdrawal. nih.gov This suggests that in certain patient groups, particularly those with underlying cardiac conditions, withdrawal may exacerbate ischemic symptoms.
However, other studies, particularly those involving patients undergoing coronary artery bypass surgery or stable patients on long-term medical therapy for angina, did not find significant early untoward effects, such as myocardial infarction, hypotension, arrhythmias, or changes in vasopressor/vasodilator requirements, following acute this compound withdrawal. nih.govjacc.orgcapes.gov.br It is noted, however, that patients with continued symptoms of rest angina may experience adverse ischemic events upon withdrawal. nih.govjacc.orgcapes.gov.br
Research also indicates that the impact of withdrawal on blood pressure can be influenced by the drug class and dose. Withdrawal of higher-dose calcium channel blockers has been associated with an increase in systolic blood pressure and reduced blood pressure control compared to usual care. frontiersin.orgconsensus.app This highlights the importance of considering the specific medication and its dosage when managing withdrawal.
While some studies suggest no "rebound" effect with this compound discontinuation, the potential for increased blood pressure or exacerbation of underlying conditions like angina underscores the importance of gradual withdrawal under medical supervision. drugs.comfda.gov This allows for monitoring of blood pressure and symptoms and provides an opportunity to manage any potential adverse effects.
The mechanism underlying potential rebound phenomena with calcium channel blockers is thought to involve the body's adaptation to the blockade of calcium channels. droracle.ai Sudden removal of the blockade could lead to a temporary overactivity of these channels, potentially resulting in vasoconstriction and increased blood pressure.
Further research, particularly larger and more appropriately powered studies, is needed to fully elucidate the incidence, severity, and predictors of withdrawal effects, including rebound hypertension, associated with this compound and other calcium channel blockers in different patient populations. frontiersin.org
Here is a summary of findings from selected studies on this compound withdrawal:
| Study Population | This compound Regimen (if specified) | Key Findings on Withdrawal | Citation |
| Patients with stable angina pectoris | 20 mg four times daily for 5 weeks | Rebound decrease in exercise tolerance and increase in exercise-induced myocardial ischemia on day 1. | nih.gov |
| Patients undergoing coronary artery bypass surgery | Not specified | No significant difference in perioperative adverse events (MI, hypotension, arrhythmias) compared to placebo withdrawal. | nih.govjacc.org |
| Stable patients on long-term medical therapy | Not specified | No early adverse effects observed in stable patients. Patients with continued rest angina may experience adverse ischemic events. | nih.govjacc.orgcapes.gov.br |
| Patients with hypertension (exploratory analysis) | Higher vs. Lower Dose | Withdrawal of higher dose calcium channel blockers associated with increased SBP and reduced BP control. | frontiersin.orgconsensus.app |
| Hypertensive patients (meta-analysis) | Various | Higher withdrawal rates due to ADRs, primarily sympathetic stimulation symptoms (tachycardia, palpitations, dizziness, headache, sweating). | ahajournals.org |
Mechanisms of Resistance and Therapeutic Tolerance to Nifedipine
Adaptive Cardiovascular Responses to Chronic Nifedipine Therapy
Chronic administration of this compound can induce a range of adaptive responses within the cardiovascular system that may contribute to altered therapeutic efficacy over time. Acutely, the rapid vasodilation caused by this compound can trigger a baroreflex-mediated increase in sympathetic tone, leading to reflex tachycardia and an increase in cardiac index. researchgate.netahajournals.org However, with chronic therapy, there is evidence suggesting that this reflex sympathetic activation may become attenuated, potentially due to resetting of the baroreflexes. ahajournals.org
Cellular and Molecular Adaptations Leading to Reduced Efficacy
At the cellular and molecular levels, several mechanisms have been proposed to explain the potential for reduced efficacy of this compound over time. This compound's action is dependent on blocking L-type calcium channels. bauschhealth.compfizer.compatsnap.comdrugbank.comphysio-pedia.comresearchgate.net One hypothesized adaptive mechanism involves changes in the expression or function of these calcium channels. Research using human induced pluripotent stem cell-derived cardiomyocytes suggests that a reduction in intracellular calcium levels induced by calcium channel blockers like this compound could trigger an upregulation of calcium ion channels. biorxiv.orgresearchgate.net This compensatory increase in channel expression might counteract the blocking effect of the drug, thereby attenuating its efficacy. biorxiv.orgresearchgate.net
Further support for this concept comes from studies in rabbits, where chronic this compound treatment was shown to induce an upregulation of functional active calcium channels in cardiac muscle membranes. This was evidenced by an increase in the maximal binding capacity (Bmax) of dihydropyridine receptors in cardiac membranes of treated animals compared to controls. nih.gov Such an increase in the number of target channels could contribute to a diminished cardiac inotropic response to this compound observed in these chronically treated animals. nih.gov
Clinical Observations of Tolerance Development
Clinical observations have indicated that therapeutic tolerance to this compound can develop in some patients, particularly in the management of stable angina pectoris. Studies involving sustained administration of this compound have reported a substantial attenuation of its anti-ischemic and anti-anginal effects over time. nih.govnih.gov For instance, in a study of patients with stable angina, the duration of the anti-ischemic effect after acute this compound administration was significantly longer than during sustained treatment. nih.govnih.gov In some individuals, a nearly complete loss of this compound efficacy was observed. nih.gov
Nifedipine and Calcium Channelopathies: Genetic and Phenotypic Research
Role of Nifedipine in Modulating Calcium Channelopathies in Neurological Disorders
Calcium channelopathies contribute to the pathophysiology of a range of neurological conditions, including migraine, epilepsy, cerebellar ataxia, and neurodevelopmental disorders openneurologyjournal.commdpi.com. This compound, primarily known for its cardiovascular applications due to its action on L-type calcium channels in smooth muscle and cardiac cells, has also been investigated for its effects in the nervous system researchgate.netnih.gov.
LTCCs, particularly CaV1.2 and CaV1.3 subtypes, are present in neurons and are involved in regulating neuronal activity and gene expression researchgate.netresearchgate.net. Studies have explored the impact of this compound on neuronal calcium influx and excitability in the context of neurological disorders. For instance, research on cortical neurons has shown that this compound can significantly reduce membrane depolarization and intracellular calcium elevation induced by oxygen/glucose deprivation, an in vitro model of cerebral ischemia ahajournals.org. This suggests a role for LTCCs in the early events of energy depletion during ischemia and indicates that dihydropyridines like this compound might ameliorate ischemic calcium accumulation ahajournals.org.
In the context of epilepsy, studies on human hypothalamic hamartoma tissue, which is associated with gelastic seizures, have demonstrated that this compound can attenuate network activity nih.gov. This compound reduced the number and duration of high-frequency oscillations (HFOs) and dampened spontaneous firing in this tissue nih.gov. The efficacy of this compound appeared enhanced under depolarized conditions, potentially relevant during seizure activity nih.gov.
While this compound's primary mechanism in the nervous system is through blocking L-type calcium channels, some research suggests it may have additional effects. One study reported that this compound induced a significant increase in spontaneous glutamate release in a calcium-independent manner, an effect not replicated by other dihydropyridines nih.gov. This finding suggests that some neurological effects or potential adverse reactions of this compound might be independent of its canonical L-type calcium channel blockade nih.gov.
Research on this compound's Effects on Specific Calcium Channel Mutations
Genetic mutations in calcium channel genes are directly responsible for various neurological channelopathies openneurologyjournal.commdpi.com. Research has begun to investigate the effects of this compound on the function of specific mutant calcium channels implicated in these disorders.
Mutations in the CACNA1D gene, which encodes the CaV1.3 L-type calcium channel, have been linked to a syndrome involving primary aldosteronism, seizures, and neurological abnormalities (PASNA) nih.govmdpi.com. These mutations often result in a gain-of-function of the CaV1.3 channel, leading to enhanced calcium entry nih.gov. Case reports have explored the use of this compound in patients with CACNA1D mutations. In one case, a patient with a de novo CACNA1D missense mutation (p.L271H) presented with congenital hyperinsulinism, primary hyperaldosteronism, and hypotonia nih.gov. Treatment including this compound was associated with improvement in muscle tone, suggesting a potential benefit for neurological symptoms in selected patients with CACNA1D gain-of-function mutations nih.gov. However, research on other CACNA1D gain-of-function variants, such as p.(G1169D), has shown retained sensitivity to other dihydropyridines like isradipine in vitro, but no conclusive beneficial effects of this compound or isradipine treatment on neurological or behavioral disorders in reported cases neurology.orgfrontiersin.org. This highlights the variable response to this compound depending on the specific mutation neurology.orgfrontiersin.org.
Mutations in the CACNA1C gene, encoding the CaV1.2 L-type calcium channel, are associated with various neurodevelopmental and neuropsychiatric disorders, including autism spectrum disorder, bipolar disorder, and schizophrenia researchgate.nettandfonline.com. The Timothy syndrome, a rare and severe disorder, is caused by a de novo gain-of-function mutation in CACNA1C researchgate.netahajournals.org. Studies using model systems, such as C. elegans with a mutation equivalent to the Timothy syndrome mutation, have shown that this gain-of-function alteration can disrupt neuronal development and behavior researchgate.net. While this compound is a dihydropyridine blocker of CaV1.2 channels, the effectiveness of this compound in addressing the neurological manifestations of specific CACNA1C mutations requires further investigation researchgate.netahajournals.org. Research on a CACNA1C pore-localizing missense mutation (E1115K) associated with cardiac issues and autism spectrum disorder showed that this mutation converted the channel into a nonselective cation channel, and the resulting inward sodium and outward potassium currents were significantly blocked by this compound in vitro nih.gov.
Mutations in the CACNA1A gene, encoding the CaV2.1 P/Q-type calcium channel, are linked to neurological disorders such as familial hemiplegic migraine (FHM1), episodic ataxia type 2 (EA2), and spinocerebellar ataxia type 6 (SCA6) mdpi.compediatricstrokejournal.comfrontiersin.org. While CaV2.1 is not an L-type channel and thus not a primary target of this compound, understanding the broader context of calcium channelopathies is relevant. Some research suggests that calcium channel blockers, including verapamil (a non-dihydropyridine calcium channel blocker), have shown some effectiveness in preventing episodes in patients with CACNA1A mutations pediatricstrokejournal.comcacna1a.orgahajournals.org. However, the direct effects of this compound on specific CACNA1A mutations and associated neurological phenotypes are less established compared to its effects on LTCCs pediatricstrokejournal.com.
Mutations in CACNA1S, encoding the CaV1.1 L-type calcium channel predominantly found in skeletal muscle, are associated with conditions like hypokalemic periodic paralysis and malignant hyperthermia susceptibility mdpi.compnas.orggenecards.org. While primarily affecting muscle function, there can be neurological implications in some of these disorders mdpi.com. Research has explored the potential of this compound in treating myotonia in myotonic dystrophy, a condition where abnormal calcium transport may be involved researchgate.net. A study showed that this compound treatment led to significant improvement in myotonia in some patients researchgate.net.
The response to this compound can vary depending on the specific calcium channel subtype and the nature of the mutation (gain-of-function vs. loss-of-function) neurology.orgcacna1a.org. Further detailed research, including electrophysiological studies of mutant channels and in vivo models, is crucial to fully understand the potential therapeutic role of this compound in specific calcium channelopathies affecting the nervous system.
Table: Compound Names and PubChem CIDs
| Compound Name | PubChem CID |
| This compound | 4485 |
Data Table: Summary of Research Findings on this compound and Calcium Channel Mutations in Neurological Contexts
| Calcium Channel Gene/Subunit | Associated Neurological Disorders/Phenotypes | Relevant Mutation(s) (Examples) | Observed Effect of this compound (or related DHP) | Research Context | Source |
| CACNA1D (CaV1.3) | PASNA syndrome (Primary Aldosteronism, Seizures, Neurological Abnormalities), Autism Spectrum Disorder, Developmental Delay, Hypotonia | p.L271H, p.(G1169D) | Associated with improved muscle tone in one case (p.L271H). nih.gov No conclusive beneficial effects on neurological/behavioral disorders for p.(G1169D) despite in vitro sensitivity to isradipine. neurology.orgfrontiersin.org | Human case reports, In vitro electrophysiology | nih.govnih.govmdpi.comneurology.orgfrontiersin.org |
| CACNA1C (CaV1.2) | Timothy Syndrome, Autism Spectrum Disorder, Neurodevelopmental Disorders | Timothy syndrome mutation (gain-of-function), E1115K | Dihydropyridines affect neuronal development in models. tandfonline.com E1115K-induced currents blocked by this compound in vitro. nih.gov | Model systems (C. elegans), In vitro electrophysiology, Research reviews | researchgate.nettandfonline.comahajournals.orgnih.gov |
| CACNA1A (CaV2.1) | Familial Hemiplegic Migraine (FHM1), Episodic Ataxia Type 2 (EA2), SCA6 | Various missense and repeat expansion mutations | Calcium channel blockers (like verapamil) show some effectiveness in preventing episodes in some cases. pediatricstrokejournal.comcacna1a.orgahajournals.org Direct effects of this compound less established. | Human case reports, Research reviews | mdpi.compediatricstrokejournal.comfrontiersin.orgcacna1a.orgahajournals.org |
| CACNA1S (CaV1.1) | Hypokalemic Periodic Paralysis, Malignant Hyperthermia Susceptibility, Myotonia in Myotonic Dystrophy | Various mutations | Showed significant improvement in myotonia in patients with myotonic dystrophy. researchgate.net | Human clinical study (myotonic dystrophy) | mdpi.comgenecards.orgresearchgate.net |
| L-type channels (general) | Cerebral ischemia (in vitro model), Hypothalamic Hamartoma (epilepsy model) | Not mutation-specific in this context | Reduced membrane depolarization and calcium elevation in ischemic model. ahajournals.org Attenuated network activity in epileptic tissue. nih.gov | In vitro studies (neuronal cultures, tissue slices) | ahajournals.orgnih.gov |
Nifedipine Formulation Science and Drug Delivery Research
Development and Evaluation of Prolonged/Extended-Release Formulations
The development of prolonged or extended-release (ER) formulations of nifedipine is a significant area of research aimed at overcoming the limitations of immediate-release forms, such as frequent dosing and potential fluctuations in plasma drug concentration. ijrpr.comijddr.in Extended-release systems are designed to maintain therapeutic drug levels over an extended period, typically 12 to 24 hours, thereby potentially improving patient compliance and providing a smoother pharmacokinetic profile. ijrpr.comijddr.inecrjournal.com
Various approaches have been explored for creating this compound ER formulations, with matrix tablets being a commonly investigated method due to their technological simplicity and cost-effectiveness. ijrpr.com Hydrophilic polymers, such as hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose, and hydroxyethyl cellulose, are frequently employed as matrix materials to control the drug release rate. ijrpr.com Studies have shown that the concentration and type of polymer significantly influence the release profile of this compound from these matrix systems. ijrpr.com For instance, formulations utilizing HPMC have demonstrated the ability to extend this compound release for up to 12 hours. ijrpr.com
Another notable extended-release technology is the Gastrointestinal Therapeutic System (GITS) formulation. This system utilizes an osmotic pump mechanism to deliver this compound at a constant rate over an extended period, typically 18-22 hours, resulting in a smooth plasma concentration-time profile. ecrjournal.com The GITS formulation consists of a two-layer core containing this compound and an osmotic polymer, surrounded by a semi-permeable membrane with a laser-drilled orifice. ecrjournal.com Water absorption into the core drives the release of the drug suspension through the orifice. ecrjournal.com In vitro dissolution studies have shown that the release from the GITS formulation is largely independent of pH and agitation, contributing to its predictable in vivo behavior. ecrjournal.com
Research also includes the development of gastroretentive drug delivery systems for this compound, such as floating matrix tablets. ijpsonline.com These systems are designed to remain in the stomach for a prolonged period, allowing for extended drug release in the upper gastrointestinal tract, which can enhance bioavailability. ijpsonline.com Studies involving polymers like Okra gum and HPMC K4M in floating matrix tablets have shown sustained release of this compound over 12 hours, with formulations exhibiting satisfactory swelling indices and zero-order release kinetics. ijpsonline.com
Data from research on prolonged-release matrix tablets highlights the impact of polymer concentration on drug release.
| Formulation | Polymer Type | Polymer Concentration | Drug Release at 12 hours (%) |
| CF4 | HPMC | 1:2 ratio | Controlled release for 12 hours ijrpr.com |
| F8 | HPMC K4M and Okra gum | Combination | 91.30 ± 0.35 ijpsonline.com |
Studies evaluating different polymer combinations and ratios are crucial in optimizing the release profile for desired therapeutic outcomes.
Research into Novel Delivery Systems (e.g., Proniosomes, Liquisolid Tablets)
Research into novel drug delivery systems for this compound explores alternative approaches to improve its solubility, bioavailability, and targeted delivery, moving beyond conventional oral formulations. Proniosomes and liquisolid tablets are among the innovative techniques being investigated.
Proniosomes are dry, free-flowing powders that, upon hydration, form niosomes, which are non-ionic surfactant vesicles. researchgate.net This system offers advantages such as improved stability compared to conventional niosome dispersions and convenience in handling and storage. Proniosomes have been explored for transdermal delivery of this compound as an alternative route to overcome the challenges of oral administration, such as extensive first-pass metabolism. nih.govtandfonline.comresearchgate.net Studies have demonstrated that this compound loaded proniosomes can achieve better permeability and sustained release through the skin compared to the pure drug. nih.govtandfonline.comresearchgate.net Research has focused on optimizing proniosome composition, including the ratio of surfactant, lecithin, and aqueous phase, to achieve desired vesicle size, entrapment efficiency, and release characteristics. nih.govtandfonline.comindexcopernicus.com For example, a formulation containing Span-40, lecithin, isopropyl alcohol, and HPMC showed promising results in terms of vesicle size and entrapment efficiency. nih.gov In vitro release studies from optimized proniosome formulations have revealed an extended release pattern. indexcopernicus.com
Liquisolid compacts represent another promising technique to enhance the dissolution rate and, consequently, the oral bioavailability of poorly water-soluble drugs like this compound. ijpsjournal.compharmaexcipients.comijper.orgresearchgate.netresearchgate.netzenodo.org This technique involves converting a liquid medication (drug dissolved or suspended in a non-volatile solvent) into a free-flowing, compressible powder by mixing it with a carrier and coating material. ijpsjournal.compharmaexcipients.comijper.orgnih.gov The drug is held within the porous structure of the carrier particles, and the non-volatile solvent increases the wetting properties and the surface area of the drug available for dissolution. pharmaexcipients.comijper.orgresearchgate.netresearchgate.netzenodo.org Studies have shown that liquisolid tablets of this compound exhibit significantly higher dissolution rates compared to conventional directly compressed tablets. researchgate.netresearchgate.netzenodo.org For instance, one study demonstrated that a liquisolid formulation achieved significantly higher drug release within the first 15 minutes compared to pure this compound or marketed tablets. pharmaexcipients.comijper.org Different carriers (e.g., Avicel PH 102) and coating materials (e.g., Aerosil 200) have been investigated, along with various drug concentrations and ratios of carrier to coating material, to optimize liquisolid formulations for enhanced this compound dissolution. pharmaexcipients.comijper.orgresearchgate.netzenodo.org
Research findings on liquisolid formulations illustrate the improvement in dissolution rate:
| Formulation Type | Drug Release at 15 minutes (%) |
| Selected Liquisolid Batch (F1) | 85.81 ± 1.0 pharmaexcipients.comijper.org |
| Pure Drug | Lower pharmaexcipients.comijper.org |
| Marketed Tablets | Lower pharmaexcipients.comijper.org |
Further research explores buccoadhesive liquisolid systems for this compound, aiming for improved bioavailability through buccal absorption. nih.gov These formulations incorporate mucoadhesive polymers to ensure retention in the buccal cavity. nih.gov
Bioequivalence Studies of Generic and Innovator Formulations
Bioequivalence studies are critical in determining whether a generic drug product performs similarly to an innovator product in terms of the rate and extent of drug absorption. juniperpublishers.comdovepress.comijtinnovation.com For prolonged or extended-release formulations of this compound, demonstrating bioequivalence is particularly important due to the modified release characteristics and the potential impact on the plasma concentration-time profile. dovepress.comgeneesmiddeleninformatiebank.nl Regulatory guidelines typically require that the pharmacokinetic parameters, such as the area under the plasma concentration-time curve (AUC) and peak plasma concentration (Cmax), of the generic formulation fall within a specified acceptance range (commonly 80% to 125%) relative to the innovator product. geneesmiddeleninformatiebank.nl
Studies comparing generic extended-release this compound formulations with the innovator product (such as Adalat OROS or Adalat LA) have been conducted to assess their bioequivalence. dovepress.comgeneesmiddeleninformatiebank.nl These studies often involve administering single or multiple doses of the test (generic) and reference (innovator) products to healthy volunteers under fasted and/or fed conditions and measuring this compound plasma concentrations over time. geneesmiddeleninformatiebank.nl
While some studies have indicated that certain generic extended-release this compound formulations may be bioequivalent to the innovator product under specific conditions, others have highlighted potential differences, particularly when administered after a meal. ecrjournal.comdovepress.comgeneesmiddeleninformatiebank.nl Variations in formulation design and manufacturing methods between generic and innovator products can lead to differences in drug release rates and subsequent pharmacokinetic profiles. juniperpublishers.comdovepress.com For instance, studies have shown that some generic modified-release this compound formulations may exhibit a more rapid increase in plasma concentration compared to the smoother profile of the GITS formulation, potentially leading to different physiological responses. ecrjournal.comdovepress.com
Bioequivalence is typically assessed by comparing pharmacokinetic parameters.
| Parameter | Acceptance Range (90% CI) |
| AUC0-t | 0.80 – 1.25 ijtinnovation.comgeneesmiddeleninformatiebank.nl |
| AUC0-∞ | 0.80 – 1.25 ijtinnovation.com |
| Cmax | 0.80 – 1.25 ijtinnovation.comgeneesmiddeleninformatiebank.nl |
Some studies have found that while AUC and Cmax values for generic this compound formulations may fall within the bioequivalence range, other parameters like minimum plasma concentration (Cmin) or the fluctuation index might show greater variability or fall outside the standard range, which could be attributed to the characteristics of prolonged-release formulations. geneesmiddeleninformatiebank.nl The impact of food on the absorption of this compound from different prolonged-release formulations is also a critical aspect evaluated in bioequivalence studies, as food can influence the rate and extent of absorption. geneesmiddeleninformatiebank.nl
Research underscores the importance of rigorous bioequivalence testing for generic extended-release this compound products to ensure their interchangeability with innovator formulations and predictable clinical performance. juniperpublishers.comdovepress.com
Analytical Methodologies for Nifedipine Quantification in Research
High-Performance Liquid Chromatography (HPLC) and its Variants (e.g., RP-HPLC, LC-MS/MS)
High-Performance Liquid Chromatography (HPLC), particularly in its reversed-phase (RP-HPLC) mode, is a widely used technique for the determination of nifedipine due to its ability to separate this compound from potential impurities and matrix components. wisdomlib.orgakjournals.comsemanticscholar.org UV detection is commonly employed with HPLC for this compound analysis, typically at wavelengths around 235 nm, 238 nm, or 326 nm, depending on the mobile phase composition and the specific method developed. derpharmachemica.comwisdomlib.orgakjournals.compensoft.net
RP-HPLC methods for this compound quantification often utilize C18 columns. akjournals.comsemanticscholar.orgnih.gov Mobile phases typically consist of mixtures of acetonitrile or methanol with water or buffer solutions, such as phosphate buffer. derpharmachemica.comakjournals.comsemanticscholar.orgpensoft.netnih.gov The pH and the ratio of organic modifier to aqueous phase are optimized to achieve adequate separation and peak shape. akjournals.comnih.gov
For enhanced sensitivity and selectivity, especially in complex biological matrices, HPLC is coupled with mass spectrometry (MS), forming LC-MS or LC-MS/MS. semanticscholar.orgdergipark.org.trthieme-connect.combiointerfaceresearch.comnih.gov LC-MS/MS is considered a method of choice in bioanalytical laboratories for its ability to quantify this compound at very low concentrations. biointerfaceresearch.comresearchgate.net Atmospheric Pressure Chemical Ionization (APCI) is one type of interface used in LC-MS/MS for this compound analysis. nih.gov Multiple Reaction Monitoring (MRM) is often used in LC-MS/MS to improve specificity by monitoring specific parent and fragment ions of this compound. thieme-connect.com
Research findings highlight the effectiveness of HPLC and its variants:
An RP-HPLC method for this compound determination in dosage forms, human milk, and urine used a C18 column with a mobile phase of phosphate buffer and acetonitrile (60:40 v/v, pH 3.0) and UV detection at 242 nm. This method showed linearity in the range of 2–12 µg/ml. Another RP-HPLC method for human milk and urine used acetonitrile and achieved recoveries between 98.9% and 101.3% in milk and 99.3% and 100.3% in urine.
An RP-HPLC method developed for this compound in proniosomal formulations, bulk drug, and marketed tablets utilized a C18 column with a mobile phase of acetonitrile and 0.1% TEA (pH 7.4) (78:22 v/v) and detection at 326 nm. The linear range was 625 to 10000 ng/mL. derpharmachemica.com
A validated RP-HPLC method for this compound in bulk and pharmaceutical formulations used an Intersil C18 column with a mobile phase of Tetra Butyl Ammonium (TBH) and Acetonitrile (25:75 v/v) and UV detection at 238 nm. Linearity was observed from 2-10 µg/ml with a correlation coefficient of 0.999. wisdomlib.org
An HPLC-MS/MS method for this compound in human plasma achieved a lower limit of quantification (LLOQ) of 0.17 ng/mL and linearity from 0.17 to 102 ng/mL. thieme-connect.com Another LC-MS/MS method reported an LLOQ of 1.01 ng/mL in human plasma with a linear range of 1-130 ng/mL. biointerfaceresearch.cominnovareacademics.in
LC-MS/MS methods have also been developed for the simultaneous determination of this compound and its metabolite, dehydrothis compound, in human plasma, demonstrating linearity in the range of 0.5-100 ng/ml for both compounds. nih.gov
Data from research studies demonstrating the performance of HPLC-based methods are presented in the table below:
| Method Type | Matrix | Column Type | Mobile Phase | Detection | Linear Range (Analyte) | LLOQ (Analyte) | Recovery (%) (Analyte) | Reference |
| RP-HPLC | Human Milk, Urine | C18 | Phosphate buffer:Acetonitrile (60:40 v/v, pH 3.0) | UV (242 nm) | 2 – 12 µg/ml | - | 98.9-101.3 (Milk), 99.3-100.3 (Urine) | |
| RP-HPLC | Proniosomes, Bulk, Tablets | ODS (C18) | Acetonitrile:0.1% TEA (pH 7.4) (78:22 v/v) | UV (326 nm) | 625 – 10000 ng/mL | 703.12 ng/mL | - | derpharmachemica.com |
| RP-HPLC | Bulk, Formulations | Intersil C18 | Tetra Butyl Ammonium:Acetonitrile (25:75 v/v) | UV (238 nm) | 2 – 10 µg/ml | 0.435 µg/ml | 99.7-100.27 | wisdomlib.org |
| HPLC-MS/MS | Human Plasma | - | - | MS/MS | 0.17 – 102 ng/mL | 0.17 ng/mL | 78.05-82.88 | thieme-connect.com |
| LC-MS/MS | Human Plasma | - | - | MS/MS | 1 – 130 ng/mL | 1.01 ng/mL | 104.1-108.7 | biointerfaceresearch.com |
| LC-MS/MS | Human Plasma | RP-18 | Methanol:50 mM ammonium acetate (50:50, v/v) | MS/MS (APCI) | 0.5 – 100 ng/ml (NIF & DNIF) | - | 95 ± 2 (NIF), 95 ± 4 (DNIF) | nih.gov |
Spectrophotometric and Electroanalytical Techniques (e.g., Voltammetry)
Spectrophotometric methods offer simpler and more economical approaches for this compound determination, primarily in pharmaceutical formulations, although applications in biological matrices have also been explored. dergipark.org.trptbioch.edu.plpjmhsonline.comnih.govsysrevpharm.orgbibliotekanauki.plekb.eg These methods often rely on the formation of colored products through reactions involving the nitro group of this compound or its oxidation/reduction. ptbioch.edu.plnih.govsysrevpharm.orgbibliotekanauki.plekb.eg UV-Visible spectrophotometry is commonly used, measuring absorbance at specific wavelengths corresponding to the formed chromophore. pjmhsonline.comekb.eg Derivative spectrophotometry has also been applied to resolve overlapping spectra and improve selectivity. dergipark.org.trptbioch.edu.pl
Examples of spectrophotometric methods include:
Methods based on the reaction of this compound's nitro group with potassium hydroxide in dimethyl sulfoxide (DMSO), producing a colored product absorbing at 430 nm. ptbioch.edu.plnih.govbibliotekanauki.pl
Methods involving the oxidation of this compound with ammonium molybdate, leading to the formation of molybdenum blue, measured at 830 nm. ptbioch.edu.plnih.govbibliotekanauki.pl
A method based on the reaction between reduced this compound and diazotized sulfamethoxazole, forming a colored product with maximum absorbance at 496 nm. pjmhsonline.com
A cloud-point extraction technique coupled with spectrophotometry, where a colored product from the reaction of reduced this compound and 4-aminoantipyrine is extracted and measured at 554 nm. sysrevpharm.org
A method utilizing proton transfer between this compound and 3,5-dinitrosalicylic acid (DNSA) in a basic medium, yielding a colored product absorbing at 359 nm. ekb.eg
Electroanalytical techniques, particularly voltammetry, have also been investigated for the determination of this compound. dergipark.org.trnih.govcdnsciencepub.comniscpr.res.inresearchgate.net These methods exploit the electrochemical behavior of this compound, which can undergo reduction or oxidation at an electrode surface. nih.govcdnsciencepub.comniscpr.res.in Voltammetric techniques such as cyclic voltammetry (CV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV) are used to study the electrochemical properties and quantify this compound. nih.govcdnsciencepub.comniscpr.res.inresearchgate.net Different working electrodes, including glassy carbon electrodes and boron-doped diamond electrodes, have been employed. nih.govcdnsciencepub.com
Research findings on spectrophotometric and electroanalytical methods include:
Spectrophotometric methods based on reactions with potassium hydroxide or ammonium molybdate showed linearity in the range of 5.0–50.0 µg/ml and 2.5–45.0 µg/ml, respectively. ptbioch.edu.plbibliotekanauki.pl
A spectrophotometric method using diazotized sulfamethoxazole showed linearity from 2-180 µg/ml. pjmhsonline.com
A cloud-point extraction spectrophotometric method demonstrated a linear range of 0.8-45 µg/mL with a detection limit of 0.395 µg/mL. sysrevpharm.org
A spectrophotometric method based on proton transfer with DNSA showed linearity from 1.5-12.5 ppm with an LOD of 0.359 ppm. ekb.eg
Electroanalytical studies using cyclic and linear sweep voltammetry at an activated glassy carbon electrode showed that both electroreduction and electrooxidation could be used for determination in specific concentration ranges. nih.gov
A differential pulse voltammetry method using a boron-doped diamond electrode allowed for the simultaneous determination of this compound and atenolol, with a linear range of 3.98–107 μmol L–1 for this compound and an LOD of 0.612 μmol L−1. cdnsciencepub.com
Data from research studies demonstrating the performance of spectrophotometric and electroanalytical methods are presented in the table below:
| Method Type | Principle | Detection Wavelength (nm) | Linear Range | LOD (Analyte) | Reference |
| Spectrophotometry | Reaction with KOH in DMSO | 430 | 5.0 – 50.0 µg/ml | - | ptbioch.edu.plnih.govbibliotekanauki.pl |
| Spectrophotometry | Oxidation with Ammonium Molybdate | 830 | 2.5 – 45.0 µg/ml | - | ptbioch.edu.plnih.govbibliotekanauki.pl |
| Spectrophotometry | Reaction with diazotized Sulfamethoxazole | 496 | 2 – 180 µg/ml | - | pjmhsonline.com |
| CPE-Spectrophotometry | Oxidative coupling with 4-aminoantipyrine after reduction, extracted with Triton X-114 | 554 | 0.8 – 45 µg/mL | 0.395 µg/mL | sysrevpharm.org |
| Spectrophotometry | Proton transfer with 3,5-dinitrosalicylic acid | 359 | 1.5 – 12.5 ppm | 0.359 ppm | ekb.eg |
| Voltammetry (DPV) | Electroreduction/oxidation at activated glassy carbon electrode | - | 2x10⁻⁵-6x10⁻⁴ M (reduction), 8x10⁻⁵-1x10⁻³ M (oxidation) | - | nih.gov |
| Voltammetry (DPV) | Oxidation at boron-doped diamond electrode | - | 3.98–107 μmol L⁻¹ | 0.612 μmol L⁻¹ | cdnsciencepub.com |
Development and Validation of Methods for Biological Matrices (e.g., Plasma, Urine, Milk)
The quantification of this compound in biological matrices such as plasma, urine, and milk is critical for pharmacokinetic studies, bioavailability assessments, and therapeutic drug monitoring in research. nih.gov These matrices present challenges due to their complex composition, which can interfere with the analytical measurement of the analyte. Therefore, sample preparation steps are essential to isolate and concentrate this compound while removing interfering substances. biointerfaceresearch.com
Common sample preparation techniques for biological matrices include liquid-liquid extraction (LLE) and solid-phase extraction (SPE). nih.govsemanticscholar.orgnih.govthieme-connect.comnih.govscispace.com LLE involves partitioning this compound from the biological matrix into an organic solvent. nih.govthieme-connect.com SPE uses a solid stationary phase to selectively retain this compound while the matrix components are washed away, followed by elution of the analyte. nih.govnih.govscispace.com Protein precipitation is another method used to remove proteins from plasma or urine samples before analysis, often by adding organic solvents like acetonitrile. pensoft.net
Method validation is a crucial step in developing analytical methods for biological matrices to ensure their reliability, accuracy, precision, sensitivity, and selectivity. derpharmachemica.comwisdomlib.orgthieme-connect.combiointerfaceresearch.cominnovareacademics.in Validation parameters typically assessed include linearity, accuracy, precision (intra-day and inter-day), recovery, limit of detection (LOD), limit of quantification (LLOQ), specificity, and stability of the analyte in the matrix. derpharmachemica.comwisdomlib.orgnih.govthieme-connect.combiointerfaceresearch.cominnovareacademics.inijpsonline.comresearchgate.net Guidelines from regulatory bodies like the US Food and Drug Administration (FDA) and the International Conference on Harmonisation (ICH) are often followed for method validation. derpharmachemica.comthieme-connect.combiointerfaceresearch.com
Detailed research findings on method development and validation for biological matrices include:
An RP-HPLC method for this compound in human milk and urine involved protein precipitation with acetonitrile followed by centrifugation and filtration. The method was validated for parameters including recovery and precision.
An HPLC method for this compound in human plasma utilized solid-phase extraction on C18 cartridges. The method was validated over a linear range of 5.0-200.0 ng/mL with an LLOQ of 5.0 ng/mL. nih.gov
A sensitive RP-HPLC method for this compound in plasma used a simple one-step extraction and achieved an average recovery of 77.48% with a linearity range of 6 to 200 ng/ml. ijpsonline.com
LC-MS/MS methods for this compound in human plasma often employ liquid-liquid extraction with solvents like ethyl acetate or methyl tert-butyl ether (MTBE). thieme-connect.comresearchgate.net Validation studies for these methods report high recoveries and low LLOQs in the nanogram per milliliter range, suitable for pharmacokinetic studies. thieme-connect.combiointerfaceresearch.comresearchgate.netinnovareacademics.in For instance, one LC-MS/MS method in human plasma showed extraction recoveries between 78.05% and 82.88% and matrix effects between 93.06% and 100.57%. thieme-connect.com Another LC-MS/MS method reported mean recoveries between 104.1% and 108.7% in human plasma. biointerfaceresearch.com
Validation of methods in biological matrices also includes assessing the stability of this compound under different storage conditions (e.g., freeze-thaw cycles, at room temperature, in the dark) due to its photosensitivity. nih.govpensoft.netresearchgate.net
Data tables summarizing validation parameters for methods in biological matrices:
| Method Type | Matrix | Sample Preparation | Linear Range (Analyte) | LLOQ (Analyte) | Recovery (%) | Intra-day Precision (RSD%) | Inter-day Precision (RSD%) | Reference |
| RP-HPLC | Human Milk | Protein precipitation | 2 – 12 µg/ml | - | 98.9-101.3 | - | - | |
| RP-HPLC | Human Urine | Protein precipitation | 2 – 12 µg/ml | - | 99.3-100.3 | - | - | |
| HPLC | Human Plasma | SPE (C18) | 5.0 – 200.0 ng/mL | 5.0 ng/mL | - | - | - | nih.gov |
| RP-HPLC | Human Plasma | One-step extraction | 6 – 200 ng/ml | 6 ng/ml | 77.48 | 0.8992-5.0387 | 5.67-15.58 | ijpsonline.com |
| LC-MS/MS | Human Plasma | LLE | 0.17 – 102 ng/mL | 0.17 ng/mL | 78.05-82.88 | ≤ 8.4 | ≤ 8.4 | thieme-connect.com |
| LC-MS/MS | Human Plasma | - | 1 – 130 ng/mL | 1.01 ng/mL | 104.1-108.7 | ≤ 7.4 | ≤ 7.4 | biointerfaceresearch.cominnovareacademics.in |
| LC-MS/MS | Human Plasma | SPE | 0.5 – 100 ng/ml (NIF & DNIF) | - | 95 ± 2 (NIF), 95 ± 4 (DNIF) | 2.9 (NIF), 2.2-4.7 (DNIF) | 3.0 (NIF), 2.2-4.7 (DNIF) | nih.gov |
| UPLC-MS/MS | Human Plasma | LLE (methyl tert-butyl ether) | 0.5–50 ng/mL | 0.1 ng/mL | 97.3-102.7 | - | - | researchgate.net |
| HPLC | Rat Plasma | Protein precipitation | 30 – 1000 ng/mL | 30 ng/mL | 101.89 | Meets criteria | Meets criteria | pensoft.net |
Theoretical Frameworks and Modeling in Nifedipine Pharmacology
Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling of Nifedipine Effects
PK/PD modeling plays a crucial role in characterizing the relationship between this compound plasma concentrations and its pharmacological effects, such as the reduction in blood pressure. cinc.orgresearchgate.netfrontiersin.org These models integrate pharmacokinetic data (what the body does to the drug) and pharmacodynamic data (what the drug does to the body) to provide a quantitative understanding of drug action over time. frontiersin.org
Studies have employed PK/PD modeling to investigate the concentration-effect relationships of this compound in hypertensive subjects. ahajournals.org By defining pharmacokinetic models and appropriate parameters for individual subjects, pharmacodynamic data can be fitted to effect models, such as linear models, to characterize the antihypertensive response. ahajournals.org The responsiveness to this compound can be quantified as the change in blood pressure per unit change in drug concentration. ahajournals.org
Physiologically based pharmacokinetic (PBPK) models are a type of PK modeling that incorporates detailed physiological and biochemical parameters to simulate drug behavior within the body. cinc.orgresearchgate.netfrontiersin.orgmdpi.com These models can predict PK parameters and have been used to assess the impact of factors like formulation differences and potential drug-drug interactions on this compound exposure and subsequent PD effects. researchgate.netfrontiersin.orgfrontiersin.org For instance, PBPK models have been linked to PD models to investigate whether changes in this compound exposure due to co-administration with other drugs, such as Apatinib or Ritonavir, could lead to significant changes in blood pressure. researchgate.netfrontiersin.orgresearchgate.netnih.gov
Data from PK/PD modeling studies can provide insights into the time course of drug effects and help predict responses to different formulations. frontiersin.org For example, PK and PD data from an immediate-release formulation of this compound, combined with in vitro dissolution data for a controlled-release formulation, have been used to predict the PK and PD profiles of the controlled-release version. frontiersin.org
In Silico Approaches to Predict Drug Behavior and Interactions
In silico methods, utilizing computational tools and simulations, are increasingly applied in this compound research to predict drug behavior, interactions, and inform formulation development. cinc.orgsrce.hrnih.govuchile.clresearchgate.netresearchgate.net These approaches can complement or reduce the need for extensive in vivo studies. nih.gov
Computational studies, including molecular dynamics simulations and docking analysis, are used to investigate the interactions of this compound with its targets, such as calcium channels, and with formulation components like cyclodextrins. nih.govuchile.clresearchgate.netresearchgate.netfrontiersin.org This helps in understanding the molecular basis of its activity and optimizing drug delivery systems. For example, computational studies have evaluated the interaction energies between this compound and different hydrogel structures to rationally design controlled-release formulations. nih.govuchile.cl
In silico gastrointestinal simulations, often integrated with PBPK models, can predict the in vivo dissolution and absorption profiles of this compound from different formulations under various conditions, including fed and fasted states. srce.hrresearchgate.net This is particularly valuable for poorly soluble drugs like this compound (BCS Class II). drugbank.comsrce.hrresearchgate.netnih.gov These models can quantitatively predict formulation-specific food effects on this compound pharmacokinetics. researchgate.net
Molecular docking studies have also been employed to design and evaluate novel dihydropyridine analogs based on the structure-activity relationships of this compound, predicting their binding affinity to calcium channel receptor structures. researchgate.netfrontiersin.org
Furthermore, in silico models, specifically PBPK models, are utilized to predict potential drug-drug interactions involving this compound, particularly those mediated by enzymes like CYP3A4, which is involved in this compound metabolism. researchgate.netresearchgate.netnih.govmdpi.com These models can simulate the impact of co-administered drugs or even food components, such as Piperine, on this compound exposure. mdpi.com
Systems Pharmacology Perspectives on this compound's Multi-Target Actions
Systems pharmacology provides a holistic view of drug action by integrating data from various levels of biological organization, from molecules to pathways and networks. While this compound is primarily known as an L-type calcium channel blocker, systems pharmacology approaches can help elucidate its potential multi-target actions and their implications. drugbank.comtargetmol.com
This compound has been shown to interact with targets beyond L-type calcium channels. drugbank.comtargetmol.com For instance, it can inhibit the drug efflux pump P-glycoprotein and block Kv channels. drugbank.comtargetmol.com It also influences signaling pathways, such as the phosphorylation of ERK1/2, MEK1/2, and Pyk2 in vascular smooth muscle cells. targetmol.com
Theoretical models, such as dose-dependent mathematical models, have been used to study the effects of this compound on physiological parameters like plasma renin activity, providing a theoretical framework to understand the dynamic response of biological systems to the drug. uctm.edu These models can utilize methods like phase plane analysis to study the system's behavior and determine equilibrium points. uctm.edu
The application of systems pharmacology to this compound could involve integrating data on its interactions with various ion channels, enzymes, transporters, and signaling molecules to build comprehensive models that predict its effects on complex physiological processes and potential interactions within biological networks.
Q & A
Q. What experimental methods are recommended for identifying and quantifying nifedipine in biological samples?
Thin-layer chromatography (TLC) using hexane:1,4-dioxane:2-propanol (15:5:2) as a solvent system is effective for separating this compound (Rf = 0.40) from its decomposition products (Rf = 0.57). UV light (254 nm) enables spot visualization, while HPLC with validated protocols (e.g., using DAS 2.0 software) is preferred for pharmacokinetic quantification in plasma .
Q. How should researchers design in vitro studies to assess this compound metabolism?
Use hepatic microsomes (e.g., rat liver microsomes) and CYP3A4-specific inhibitors to evaluate metabolic pathways. Measure kinetic constants (Km, Vmax) via nonlinear regression and determine inhibition constants (Ki) using Lineweaver-Burk plots. Ensure quantitation limits (e.g., 2.34 mmol/L for this compound) are validated .
Q. What are the standard protocols for ensuring reproducibility in this compound formulation studies?
Employ design-of-experiment (DoE) approaches like the Box-Behnken design to optimize polymeric microsphere formulations. Key parameters include polymer concentration, crosslinking agent ratio, and drug release kinetics. Validate responses (e.g., encapsulation efficiency) using regression analysis .
Advanced Research Questions
Q. How can researchers resolve contradictions in this compound polymorphism studies?
Polymorphic forms (α, β, γ) exhibit solvent-dependent crystallization. For example, the β form dominates in DMSO and MeCN, while γ forms arise in DMF. Use single-crystal X-ray diffraction (SCXRD) for structural confirmation and differential scanning calorimetry (DSC) to assess thermal stability. Cross-reference with CSD databases (e.g., BICCIZ06) .
Q. What methodologies are critical for assessing this compound's environmental risk?
Calculate Predicted Environmental Concentrations (PEC) using regional sales data and compare against EU action limits (0.01 µg/L). Note that insufficient aquatic toxicity data currently preclude PNEC derivation, necessitating probabilistic modeling for risk extrapolation .
Q. How do inter-individual differences impact this compound pharmacokinetics in drug interaction studies?
In vivo rat studies show dose-dependent variability (e.g., 12–57% Cmax reduction with baicalin co-administration). Use non-compartmental pharmacokinetic models (DAS 2.0 software) and stratified analyses to account for factors like CYP3A4 activity and plasma protein binding .
Q. What advanced techniques validate co-amorphous this compound systems for enhanced solubility?
Pair this compound with valsartan or famotidine and characterize via X-ray diffraction (PXRD), FTIR, and molecular dynamics simulations. Stability testing under accelerated conditions (40°C/75% RH) and in vitro dissolution profiling (pH 1.2–6.8) are essential .
Q. How can population pharmacokinetic models improve this compound dosing regimens?
Integrate covariates like age, hepatic function, and CYP3A4 polymorphisms using nonlinear mixed-effects modeling (NONMEM). Validate models with bootstrap analysis and visual predictive checks to optimize therapeutic indices .
Methodological Considerations
Q. How should researchers address conflicting data in this compound-calcium channel interaction studies?
Perform patch-clamp electrophysiology to isolate L-type channel contributions. Linear regression of plateau potential durations against this compound-sensitive components clarifies dose-response relationships. Control for voltage-gated sodium/potassium channel interference .
Q. What statistical approaches are recommended for analyzing this compound clinical trial data with small sample sizes?
Use exact tests (e.g., Fisher’s) for adverse effect comparisons and Bayesian hierarchical models to pool sparse data. Narrative synthesis is advised when heterogeneity precludes meta-analysis .
Tables for Key Data
| Parameter | Value | Reference |
|---|---|---|
| TLC Rf (this compound) | 0.40 | |
| Hepatic Metabolism (CYP3A4) | 60–80% urinary excretion | |
| Baicalin Interaction (Cmax Reduction) | 40–65% | |
| Co-amorphous Stability | >6 months (accelerated conditions) |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


