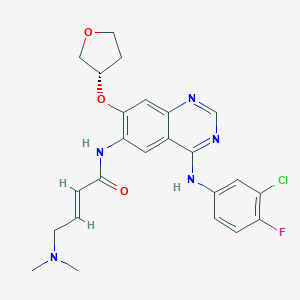
Afatinib
Overview
Description
Afatinib (GIOTRIF®) is a second-generation, irreversible tyrosine kinase inhibitor (TKI) targeting the ErbB family of receptors, including EGFR (ErbB1), HER2 (ErbB2), and ErbB4 . It covalently binds to the kinase domains of these receptors via a reactive acrylamide group, leading to sustained inhibition of downstream signaling pathways critical for cancer cell proliferation and survival . Approved for EGFR mutation-positive non-small cell lung cancer (NSCLC), this compound demonstrated superior progression-free survival (PFS) over chemotherapy in phase III trials (median PFS: 11.1 vs. 6.9 months) . Its broad-spectrum activity and irreversible binding differentiate it from first-generation EGFR TKIs like gefitinib and erlotinib .
Preparation Methods
Optimization of Reaction Conditions
Nitration Reagent Selection
Traditional nitration using nitric acid/sulfuric acid mixtures generates corrosive waste, complicating industrial-scale production. Substituting potassium nitrate in sulfuric acid improves safety and selectivity, reducing byproducts such as di-nitrated impurities .
Catalytic Systems in Reduction Steps
Comparative studies show that Pd/C (10% loading) outperforms Raney nickel in nitro-reduction, achieving >99% conversion with lower catalyst costs . Hydrogenation at 50–60 psi H₂ pressure minimizes over-reduction side reactions.
Solvent and Temperature Effects
-
Cyclization : Anhydrous ethanol vs. methanol: Ethanol provides higher yields (92% vs. 85%) due to better intermediate solubility .
-
Salification : Methanol/water mixtures (3:1 v/v) optimize this compound dimaleate crystallization, yielding 99.5% purity .
Industrial Scalability and Process Improvements
Pilot-Scale Validation
The six-step route was validated at pilot scale (10 kg batches), demonstrating consistent yields (40–42%) and impurity profiles . Key adjustments included:
-
Molar ratios : 1:1.2 (starting material to nitration reagent) to prevent excess reagent accumulation.
-
Crystallization control : Slow cooling (0.5°C/min) to minimize occluded solvents.
Cost Reduction Strategies
-
Reagent recycling : Toluene and ethanol are recovered via distillation (90% efficiency) .
-
Telescoping : Intermediate III is used directly without isolation, reducing processing time by 30% .
Impurity Control and Quality Assurance
Identified Impurities
-
Acetamide impurity : Formed during amidation (2–3% in unoptimized batches) .
-
Hydroxy impurity : Oxidation byproduct (0.5–1.2%) controlled via nitrogen sparging .
-
N-Oxide impurity : Resulting from residual peroxide in solvents, mitigated by activated carbon treatment .
Analytical Methods
-
HPLC : Gradient elution (0.1% TFA in acetonitrile/water) resolves all impurities with <0.1% limit of quantification .
-
LC-MS : Confirms impurity structures via m/z fragmentation patterns .
Comparative Analysis of Preparation Methods
Chemical Reactions Analysis
Types of Reactions
Afatinib undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions to form different metabolites.
Reduction: Reduction reactions can modify the functional groups on this compound, potentially altering its activity.
Substitution: Substitution reactions can occur at various positions on the quinazoline ring, leading to the formation of different derivatives.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Reagents like halogens and nucleophiles are used under controlled conditions to achieve substitution reactions.
Major Products
The major products formed from these reactions include various metabolites and derivatives of this compound, which may have different pharmacological properties .
Scientific Research Applications
Non-Small Cell Lung Cancer (NSCLC)
Afatinib is primarily indicated for patients with NSCLC harboring activating EGFR mutations, particularly exon 19 deletions or L858R substitutions. Clinical trials have demonstrated that this compound significantly improves progression-free survival (PFS) and overall survival (OS) compared to traditional chemotherapy .
Clinical Trials:
- LUX-Lung Trials: This series of clinical trials assessed this compound's efficacy in various settings:
- LUX-Lung 1: Compared this compound with chemotherapy in first-line treatment.
- LUX-Lung 3 & 6: Compared this compound with other EGFR inhibitors like erlotinib and gefitinib.
| Trial Name | Phase | Comparison | Outcome |
|---|---|---|---|
| LUX-Lung 1 | II | This compound vs. Chemotherapy | Improved PFS |
| LUX-Lung 3 | III | This compound vs. Erlotinib | Superior OS |
| LUX-Lung 6 | III | This compound vs. Gefitinib | Enhanced response rate |
HER2-Positive Breast Cancer
This compound is being investigated as a monotherapy for patients with HER2-positive breast cancer who have progressed on trastuzumab therapy. Although not yet FDA-approved for this indication, preliminary studies suggest potential benefits in terms of tumor response and patient outcomes .
Efficacy and Safety Profile
Meta-analyses indicate that this compound extends survival rates without significantly increasing treatment-related mortality risks . Its side effects are generally manageable and include diarrhea, rash, and stomatitis. Compared to traditional chemotherapy, this compound tends to produce fewer severe adverse effects .
Case Studies
Several case reports have illustrated the effectiveness of this compound in real-world settings:
- Case Study 1: A patient with advanced NSCLC exhibited a significant reduction in tumor size after initiating this compound therapy, demonstrating a robust response to treatment.
- Case Study 2: A patient with HER2-positive breast cancer experienced disease stabilization after switching from trastuzumab to this compound, showcasing its potential as a treatment alternative.
Mechanism of Action
Afatinib exerts its effects by irreversibly binding to and inhibiting the kinase activity of EGFR, HER2, and HER4. This inhibition prevents the autophosphorylation and subsequent activation of downstream signaling pathways that promote cell proliferation and survival. By blocking these pathways, this compound effectively inhibits the growth and spread of cancer cells .
Comparison with Similar Compounds
Gefitinib
- Mechanism : Reversible EGFR inhibitor (first-generation TKI) .
- Efficacy : In EGFR-mutant NSCLC, gefitinib showed comparable overall response rates (ORRs) to afatinib (~70%) but shorter median PFS (10.8 vs. 11.1 months) . The LUX-Lung 7 trial directly compared this compound and gefitinib, revealing longer PFS (11.0 vs. 10.9 months) and time-to-treatment failure (13.7 vs. 11.5 months) for this compound .
- Toxicity : Gefitinib has lower rates of severe diarrhea (5% vs. 14%) and paronychia (9% vs. 15%) compared to this compound .
- Cost-Effectiveness : this compound provides higher quality-adjusted life-year (QALY) gains but at a higher cost than gefitinib .
Erlotinib
- Mechanism : Reversible EGFR inhibitor with additional HER2 activity .
- Indirect comparisons suggest comparable efficacy, though this compound may offer longer PFS in exon 19 deletions .
- Toxicity : Erlotinib shares a similar toxicity profile to gefitinib but with higher rates of rash (75% vs. 70%) compared to this compound .
- Cost-Effectiveness : Erlotinib is less costly than this compound but provides fewer QALYs .
Osimertinib
- Mechanism : Third-generation irreversible EGFR TKI targeting T790M resistance mutations .
- Efficacy: Superior PFS to this compound in EGFR T790M-positive NSCLC (18.9 vs.
- Toxicity : Lower incidence of grade ≥3 adverse events (AEs) compared to this compound (34% vs. 48%) .
Lapatinib
- Mechanism : Reversible dual EGFR/HER2 inhibitor .
- Toxicity : Higher rates of hepatotoxicity than this compound .
Key Differentiators of this compound
Irreversible Binding and Broad-Spectrum Activity
This compound’s acrylamide group enables covalent binding to ErbB receptors, providing prolonged inhibition even in cells with HER2 amplification or ErbB4 activation . This contrasts with reversible inhibitors like gefitinib, which fail to inhibit T790M or HER2-driven resistance .
Efficacy in Exon 19 Deletions
In patients with exon 19 deletions, this compound achieved a median PFS of 13.6 months vs. 6.9 months for chemotherapy, outperforming gefitinib and erlotinib in this subgroup .
Adverse Event Management
While this compound has higher rates of diarrhea (95% vs. 60% for gefitinib) and stomatitis, dose reductions (to 20–30 mg/day) maintain efficacy while reducing AE severity .
Comparative Data Table
| Parameter | This compound | Gefitinib | Erlotinib | Osimertinib |
|---|---|---|---|---|
| Targets | EGFR, HER2, ErbB4 | EGFR | EGFR, HER2 | EGFR (incl. T790M) |
| Binding Type | Irreversible | Reversible | Reversible | Irreversible |
| Median PFS (months) | 11.1–13.6* | 10.8–10.9 | 9.7–12.0 | 18.9† |
| Common AEs | Diarrhea, rash, paronychia | Rash, hepatotoxicity | Rash, diarrhea | Rash, cardiotoxicity |
| Cost-Effectiveness | Higher QALYs, higher cost | Lower cost, fewer QALYs | Intermediate | Highest cost, superior in T790M |
*Exon 19 deletions; †T790M-positive patients.
Resistance and Combination Strategies
Acquired resistance to this compound involves MET amplification or epithelial-to-mesenchymal transition (EMT). Preclinical studies show combining this compound with crizotinib (MET inhibitor) or docetaxel overcomes resistance .
Biological Activity
Afatinib is an irreversible tyrosine kinase inhibitor (TKI) primarily used in the treatment of non-small cell lung cancer (NSCLC) with specific epidermal growth factor receptor (EGFR) mutations. Its biological activity is characterized by its ability to inhibit multiple members of the human epidermal growth factor receptor (HER) family, including EGFR, HER2, and HER4. This article explores this compound's biological activity, mechanisms, clinical efficacy, and relevant case studies.
This compound works by irreversibly binding to the active site of EGFR and other HER family receptors, thereby inhibiting their kinase activity. This inhibition leads to:
- Decreased Tumor Cell Proliferation : By blocking EGFR signaling pathways, this compound reduces cellular proliferation and survival.
- Induction of Apoptosis : this compound activates apoptotic pathways in cancer cells, contributing to tumor shrinkage.
- Inhibition of Angiogenesis : The drug disrupts pathways that facilitate new blood vessel formation, limiting tumor growth.
The potency of this compound varies among different mutations of the EGFR gene. It exhibits strong inhibitory effects against common mutations such as Exon 19 deletions and L858R mutations, with lower efficacy against T790M mutations associated with resistance to first-generation TKIs.
Efficacy in NSCLC
This compound has demonstrated significant clinical efficacy in patients with EGFR-mutated NSCLC. A comprehensive analysis from various clinical trials provides insights into its effectiveness:
| Study | Patient Cohort | Overall Response Rate (ORR) | Median Progression-Free Survival (PFS) | Median Overall Survival (OS) |
|---|---|---|---|---|
| LUX-Lung 1 | 345 patients with prior TKI failure | 27% | 2.9 months | 7.9 months |
| LUX-Lung 2 | 130 patients with common mutations | 56% | 11.0 months | 27.9 months |
| Non-interventional study | 152 patients with diverse mutations | 74.6% | 12.2 months | 30.4 months |
The LUX-Lung studies indicated that this compound provides superior outcomes compared to first-generation TKIs like gefitinib and erlotinib, especially in patients harboring specific mutations .
Case Study 1: Treatment Response in Brain Metastases
A patient with NSCLC who had brain metastases was treated with this compound after progression on first-line therapy. The patient exhibited a partial response after three months of treatment, achieving a quality of life improvement without significant adverse effects. This case underscores this compound's potential in managing CNS involvement in metastatic disease .
Case Study 2: Uncommon EGFR Mutations
Another case involved a patient with an uncommon EGFR mutation who received this compound as a second-line treatment after failing erlotinib. The patient achieved a partial response and maintained stable disease for over two years, highlighting this compound's efficacy beyond common mutations .
Biomarkers and Predictive Factors
Recent studies have focused on identifying biomarkers that predict response to this compound treatment:
- Phosphorylation Levels : Baseline levels of phosphorylated ERK1/2 and RB1 were correlated with metabolic responses in head and neck squamous cell carcinoma (HNSCC) patients treated with this compound .
- Tumor-Infiltrating B Cells : An increase in tumor-infiltrating B cells was observed in responders, suggesting a potential immunomodulatory effect of this compound .
- NF-kappa B Signaling : Activation of NF-kappa B signaling pathways was linked to non-responsiveness, indicating that further exploration into these pathways may provide insights into resistance mechanisms .
Safety Profile
This compound is generally well-tolerated; however, it is associated with specific adverse effects:
- Common side effects include diarrhea, rash, and stomatitis.
- Serious adverse events are rare but can include interstitial lung disease and liver function abnormalities.
Management strategies for these side effects often include dose adjustments or supportive care measures .
Q & A
Basic Research Question: How should researchers design a Phase III clinical trial to evaluate afatinib's efficacy in EGFR-mutant NSCLC?
Methodological Answer:
Phase III trials for this compound should include patients with confirmed EGFR mutations (e.g., exon 19 deletions, L858R) via validated molecular testing. Stratification factors should include mutation subtype and race (Asian vs. non-Asian) to control for genetic and ethnic heterogeneity . The comparator arm should use platinum-based chemotherapy (e.g., cisplatin/pemetrexed), with progression-free survival (PFS) as the primary endpoint assessed by independent review. Secondary endpoints should include overall survival (OS), tumor response rates, adverse event (AE) profiles, and patient-reported outcomes (PROs) such as cough, dyspnea, and pain control . For example, LUX-Lung 3 demonstrated a median PFS of 11.1 months for this compound vs. 6.9 months for chemotherapy (HR 0.58) .
Basic Research Question: What is the recommended methodology for managing this compound-related adverse events (AEs) in clinical trials?
Methodological Answer:
Proactive AE management should involve dose reductions (e.g., from 40 mg to 30 mg/day) for grade ≥3 toxicities (e.g., diarrhea, rash, stomatitis). Predefined protocols for supportive care (e.g., loperamide for diarrhea, topical steroids for rash) and regular patient monitoring (e.g., biweekly assessments during dose escalation) are critical. In the LUX-Lung 3 trial, 53.4% of patients required dose reductions due to AEs, yet PROs still favored this compound over chemotherapy . Researchers should document AE resolution timelines and correlate these with treatment interruptions to optimize tolerability .
Advanced Research Question: How can next-generation sequencing (NGS) clarify mechanisms of acquired resistance to this compound in EGFR-mutant NSCLC?
Methodological Answer:
NGS should be performed on pre-treatment and post-progression tumor samples or circulating tumor DNA (ctDNA) to identify resistance mechanisms. Key targets include EGFR T790M (detected in ~40% of this compound-resistant cases) and C797S mutations, as well as off-target alterations (e.g., BRAF V600E, MET amplification) . Liquid biopsy protocols using droplet digital PCR (ddPCR) enable real-time monitoring of T790M allele frequency, which correlates with treatment response . For example, T790M-positive patients transitioning to osimertinib had prolonged PFS (median 10.3 months vs. 1.4 months in T790M-negative cases) .
Advanced Research Question: How do researchers reconcile contradictory data on this compound's survival benefits vs. toxicity risks?
Methodological Answer:
Risk-benefit analyses should weigh PFS/OS improvements against severe AEs (e.g., 17% grade ≥3 diarrhea, 4 treatment-related deaths in this compound trials vs. none in chemotherapy arms) . Statistical models (e.g., Cox proportional hazards) should adjust for covariates like mutation subtype (Del19 patients derive greater OS benefit: HR 0.47) . Meta-analyses pooling LUX-Lung 3/6 data show a survival advantage for Del19 patients (median OS 33.3 months with this compound vs. 21.1 months with chemotherapy) . Researchers must stratify toxicity data by dose adjustments and patient subgroups (e.g., elderly populations).
Basic Research Question: What clinical endpoints are most relevant for evaluating this compound's activity against uncommon EGFR mutations?
Methodological Answer:
For uncommon mutations (e.g., G719X, S768I, L861Q), prioritize objective response rate (ORR) and disease control rate (DCR) over PFS due to smaller sample sizes. Post-hoc analyses of LUX-Lung trials reported ORRs of 60–78% for G719X/L861Q/S768I mutations . In vitro models comparing this compound with gefitinib/osimertinib should assess IC50 values; this compound showed superior potency against G719X (IC50 1.6 nM vs. 18.4 nM for osimertinib) .
Advanced Research Question: How can researchers optimize sequential therapy with this compound followed by osimertinib?
Methodological Answer:
Design observational studies tracking time on treatment (TOT), defined as the interval from this compound initiation to osimertinib discontinuation. In one study, median TOT was 27.7 months for Del19 patients . Protocolized T790M monitoring via ctDNA every 8–12 weeks enables timely transition to osimertinib. Patients with T790M/C797S co-mutations may require combination therapies (e.g., cetuximab + osimertinib), as C797S reduces osimertinib PFS (median 6.9 vs. 10.3 months in C797S-negative cases) .
Basic Research Question: What statistical methods are appropriate for meta-analyses of this compound's efficacy across trials?
Methodological Answer:
Use random-effects models to account for heterogeneity across studies (e.g., LUX-Lung 3/6 vs. LUX-Lung 7). Pool hazard ratios (HRs) for PFS/OS and odds ratios (ORs) for ORR/DCR. For safety, calculate incidence rates (e.g., 12% pooled ORR for this compound post-1st-gen TKI failure) . Funnel plots and Egger’s regression assess publication bias, while subgroup analyses explore mutation-specific effects (e.g., Del19 HR 0.47 for PFS) .
Advanced Research Question: How does this compound's irreversible ErbB inhibition influence preclinical study design?
Methodological Answer:
In vitro studies should compare this compound with reversible inhibitors (e.g., gefitinib) using cell lines expressing EGFR T790M or HER2 mutations. X-ray crystallography and mass spectrometry confirm covalent binding to EGFR/HER2 . Dose-response assays (e.g., EC50 for ErbB4 inhibition: 1 nM) and synergy studies with anti-ErbB antibodies (e.g., cetuximab) are critical for combination therapy development .
Properties
IUPAC Name |
(E)-N-[4-(3-chloro-4-fluoroanilino)-7-[(3S)-oxolan-3-yl]oxyquinazolin-6-yl]-4-(dimethylamino)but-2-enamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ULXXDDBFHOBEHA-CWDCEQMOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CC=CC(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)OC4CCOC4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(C)C/C=C/C(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)O[C@H]4CCOC4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C24H25ClFN5O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20893451 | |
| Record name | Afatinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20893451 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
485.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
850140-72-6, 439081-18-2 | |
| Record name | Afatinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=850140-72-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Afatinib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0850140726 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Afatinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08916 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Tovok | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=750691 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Afatinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20893451 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Afatinib | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AFATINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/41UD74L59M | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.