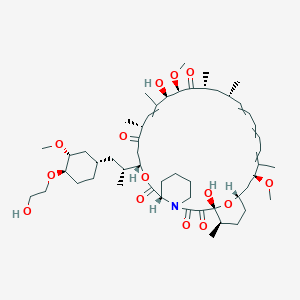
Everolimus
Description
Historical Background and Discovery
This compound traces its origins to the discovery of rapamycin (sirolimus) in 1965 from Streptomyces hygroscopicus isolated from a soil sample on Rapa Nui (Easter Island). Initial interest in rapamycin centered on its antifungal properties, but its immunosuppressive and antiproliferative effects soon became apparent. By the 1990s, structural modifications of rapamycin aimed to improve pharmacokinetics and reduce toxicity. This compound, synthesized by adding a 2-hydroxyethyl chain at the C40 position, was developed by Novartis and approved by the FDA in 2009 for renal cell carcinoma (RCC).
Classification and Relationship to Rapamycin Derivatives
This compound belongs to the rapalog family, first-generation mTOR inhibitors characterized by structural analogs of rapamycin. Key derivatives include:
- Temsirolimus : A water-soluble prodrug for intravenous use.
- Ridaforolimus : Investigated for sarcoma and other malignancies.
- This compound : Distinguished by its oral bioavailability and specificity for mTOR complex 1 (mTORC1).
Structurally, this compound (C₅₃H₈₃NO₁₄, molecular weight 958.24 g/mol) retains the macrolide core of rapamycin but incorporates a 40-O-(2-hydroxyethyl) group, enhancing solubility and stability.
Significance in Biochemical and Molecular Research
This compound inhibits mTORC1, a serine/threonine kinase regulating cell growth, protein synthesis, and autophagy. By binding FK506-binding protein 12 (FKBP12), this compound disrupts mTORC1 signaling, downregulating hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF). This mechanism has made it a cornerstone in studying mTOR-driven pathologies, including cancer, tuberous sclerosis complex (TSC), and organ transplant rejection.
Properties
Key on ui mechanism of action |
Mechanistic target of rapamycin (mTOR) is a serine-threonine kinase that functions via two multiprotein complexes, namely mTORC1 and mTORC2, each characterized by different binding partners that confer separate functions. mTORC1 function is tightly regulated by PI3-K/Akt and is sensitive to rapamycin. mTORC2 is sensitive to growth factors, not nutrients, and is associated with rapamycin-insensitivity. mTORC1 regulates protein synthesis and cell growth through downstream molecules: 4E-BP1 (also called EIF4E-BP1) and S6K. Also, mTORC2 is thought to modulate growth factor signaling by phosphorylating the C-terminal hydrophobic motif of some AGC kinases such as Akt and SGK. Recent evidence has suggested that mTORC2 may play an important role in maintenance of normal as well as cancer cells by virtue of its association with ribosomes, which may be involved in metabolic regulation of the cell. Rapamycin (sirolimus) and its analogs known as rapalogues, such as RAD001 (everolimus) and CCI-779 (temsirolimus), suppress mTOR activity through an allosteric mechanism that acts at a distance from the ATP-catalytic binding site, and are considered incomplete inhibitors. Moreover, these compounds suppress mTORC1-mediated S6K activation, thereby blocking a negative feedback loop, leading to activation of mitogenic pathways promoting cell survival and growth. Consequently, mTOR is a suitable target of therapy in cancer treatments. However, neither of these complexes is fully inhibited by the allosteric inhibitor rapamycin or its analogs. In recent years, new pharmacologic agents have been developed which can inhibit these complexes via ATP-binding mechanism, or dual inhibition of the canonical PI3-K/Akt/mTOR signaling pathway. These compounds include WYE-354, KU-003679, PI-103, Torin1, and Torin2, which can target both complexes or serve as a dual inhibitor for PI3-K/mTOR. This investigation describes the mechanism of action of pharmacological agents that effectively target mTORC1 and mTORC2 resulting in suppression of growth, proliferation, and migration of tumor and cancer stem cells. Mammalian target of rapamycin (mTOR) inhibitors have anti-tumor effects against renal cell carcinoma, pancreatic neuroendocrine cancer and breast cancer. In this study, we analyzed the antitumor effects of mTOR inhibitors in small cell lung cancer (SCLC) cells and sought to clarify the mechanism of resistance to mTOR inhibitors. We analyzed the antitumor effects of three mTOR inhibitors including everolimus in 7 SCLC cell lines by MTS assay. Gene-chip analysis, receptor tyrosine kinases (RTK) array and Western blotting analysis were performed to identify molecules associated with resistance to everolimus. Only SBC5 cells showed sensitivity to everolimus by MTS assay. We established two everolimus resistant-SBC5 cell lines (SBC5 R1 and SBC5 R10) by continuous exposure to increasing concentrations of everolimus stepwise. SPP1 and MYC were overexpressed in both SBC5 R1 and SBC5 R10 by gene-chip analysis. High expression levels of eukaryotic translation initiation factor 4E (eIF4E) were observed in 5 everolimus-resistant SCLC cells and SBC5 R10 cells by Western blotting. MYC siRNA reduced eIF4E phosphorylation in SBC5 cells, suggesting that MYC directly activates eIF4E by an mTOR-independent bypass pathway. Importantly, after reduction of MYC or eIF4E by siRNAs, the SBC5 parent and two SBC5-resistant cells displayed increased sensitivity to everolimus relative to the siRNA controls. These findings suggest that eIF4E has been shown to be an important factor in the resistance to everolimus in SCLC cells. Furthermore, a link between MYC and mTOR-independent eIF4E contribute to the resistance to everolimus in SCLC cells. Control of the MYC-eIF4E axis may be a novel therapeutic strategy for everolimus action in SCLC. Everolimus inhibits antigenic and interleukin (IL-2 and IL-15) stimulated activation and proliferation of T and B lymphocytes. In cells, everolimus binds to a cytoplasmic protein, the FK506 Binding Protein-12 (FKBP-12), to form an immunosuppressive complex (everolimus: FKBP-12) that binds to and inhibits the mammalian Target Of Rapamycin (mTOR), a key regulatory kinase. In the presence of everolimus phosphorylation of p70 S6 ribosomal protein kinase (p70S6K), a substrate of mTOR, is inhibited. Consequently, phosphorylation of the ribosomal S6 protein and subsequent protein synthesis and cell proliferation are inhibited. The everolimus: FKBP-12 complex has no effect on calcineurin activity. In rats and nonhuman primate models, everolimus effectively reduces kidney allograft rejection resulting in prolonged graft survival. Everolimus is an inhibitor of mammalian target of rapamycin (mTOR), a serine-threonine kinase, downstream of the PI3K/AKT pathway. The mTOR pathway is dysregulated in several human cancers. Everolimus binds to an intracellular protein, FKBP-12, resulting in an inhibitory complex formation with mTOR complex 1 (mTORC1) and thus inhibition of mTOR kinase activity. Everolimus reduced the activity of S6 ribosomal protein kinase (S6K1) and eukaryotic initiation factor 4E-binding protein (4E-BP1), downstream effectors of mTOR, involved in protein synthesis. S6K1 is a substrate of mTORC1 and phosphorylates the activation domain 1 of the estrogen receptor which results in ligand-independent activation of the receptor. In addition, everolimus inhibited the expression of hypoxia-inducible factor (e.g., HIF-1) and reduced the expression of vascular endothelial growth factor (VEGF). Inhibition of mTOR by everolimus has been shown to reduce cell proliferation, angiogenesis, and glucose uptake in in vitro and/or in vivo studies. Constitutive activation of the PI3K/Akt/mTOR pathway can contribute to endocrine resistance in breast cancer. In vitro studies show that estrogen-dependent and HER2+ breast cancer cells are sensitive to the inhibitory effects of everolimus, and that combination treatment with everolimus and Akt, HER2, or aromatase inhibitors enhances the anti-tumor activity of everolimus in a synergistic manner. Two regulators of mTORC1 signaling are the oncogene suppressors tuberin-sclerosis complexes 1 and 2 (TSC1, TSC2). Loss or inactivation of either TSC1 or TSC2 leads to activation of downstream signaling. In TSC, a genetic disorder, inactivating mutations in either the TSC1 or the TSC2 gene lead to hamartoma formation throughout the body. |
|---|---|
CAS No. |
159351-69-6 |
Molecular Formula |
C53H83NO14 |
Molecular Weight |
958.2 g/mol |
IUPAC Name |
(1R)-1,18-dihydroxy-12-[1-[4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone |
InChI |
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/t32?,34?,35?,36?,38?,39?,40?,41?,43?,44?,45?,46?,48?,49?,53-/m1/s1 |
InChI Key |
HKVAMNSJSFKALM-VUDSBINYSA-N |
SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Isomeric SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)[C@@]1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC |
Appearance |
White to off-white solid powder |
Key on ui application |
Everolimus is currently used as an immunosuppressant to prevent rejection of organ transplants. In a similar fashion to other mTOR inhibitors Everolimus' effect is solely on the mTORC1 protein and not on the mTORC2 protein. |
boiling_point |
998.7±75.0 °C at 760 mmHg |
melting_point |
N/A |
Other CAS No. |
159351-69-6 |
Pictograms |
Health Hazard |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
Stable under recommended storage conditions. |
solubility |
Soluble in DMSO, not in water |
storage |
−20°C |
Synonyms |
001, RAD 40-O-(2-hydroxyethyl)-rapamycin Afinitor Certican everolimus RAD 001 RAD, SDZ RAD001 SDZ RAD SDZ-RAD |
Origin of Product |
United States |
Preparation Methods
Synthesis of the Triflate Intermediate
The preparation of this compound begins with the synthesis of a silyl-protected triflate intermediate, critical for alkylating rapamycin. A representative method involves reacting 2-([trisubstituted]silyloxy)ethanol with trifluoromethanesulfonic anhydride (Tf₂O) in the presence of a base such as 2,6-lutidine. For instance, t-butyldimethylsilyloxyethanol reacts with Tf₂O at -40°C in dichloromethane (DCM) to yield 2-(t-butyldimethylsilyloxy)ethyl triflate (Formula IVa). This intermediate is highly reactive, necessitating inert conditions and low temperatures to prevent hydrolysis.
Table 1: Reaction Conditions for Triflate Intermediate Synthesis
Alkylation of Sirolimus
The triflate intermediate reacts with sirolimus (rapamycin) to form a protected this compound derivative. This step requires a base to deprotonate sirolimus’s C-40 hydroxyl group, facilitating nucleophilic substitution. Silver acetate (AgOAc) is often added to enhance reactivity, as demonstrated in Example 1 of Patent WO2016020664A1. Sirolimus dissolved in DCM is treated with the triflate intermediate at -40°C, followed by gradual warming to 40°C. The use of 2,6-lutidine as both a base and catalyst ensures high regioselectivity, yielding 40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin (TBS-everolimus) with 60–85% purity.
Critical Factor : Omitting AgOAc (as in Example 2 of the same patent) reduces purity to 10–20%, underscoring the metal’s role in stabilizing the transition state.
Deprotection to Yield this compound
The final step involves cleaving the silyl protecting group using aqueous HCl in methanol. TBS-everolimus is stirred with 1N HCl at pH 1–3, resulting in quantitative deprotection to this compound. Post-reaction, the crude product is extracted with ethyl acetate and purified via preparative HPLC to achieve >99% purity.
Table 2: Deprotection Conditions and Outcomes
| Protected Intermediate | Deprotection Agent | Solvent | Temperature | Purity After HPLC (%) |
|---|---|---|---|---|
| TBS-everolimus | 1N HCl | Methanol | 25°C | >99 |
| Diphenyl-tert-butylsilyl-everolimus | HF·Pyridine | THF | 0°C | 95 |
Optimization of Reaction Conditions
Solvent and Base Selection
Early methods used DCM for triflate synthesis but faced challenges in large-scale operations due to solvent volatility. Substituting DCM with n-heptane improved stability and facilitated easier filtration of byproducts. Similarly, replacing pyridine with 2,6-lutidine reduced side reactions, as lutidine’s steric hindrance minimizes over-alkylation.
Temperature Control
Maintaining temperatures below -20°C during triflate formation is crucial to prevent decomposition. However, the alkylation step benefits from a gradual temperature increase (from -40°C to 40°C), enhancing reaction kinetics without compromising yield.
Large-Scale Manufacturing Processes
Industrial-scale production (Example 3, WO2016020664A1) employs toluene as the solvent for sirolimus alkylation, enabling higher throughput compared to DCM. The process involves:
-
Batchwise triflate addition : Three portions of TBS-glycol-triflate are added to sirolimus at 60–65°C, achieving 60–70% conversion.
-
Solvent switching : Replacing DCM with n-heptane simplifies byproduct removal via filtration.
-
One-pot deprotection : Crude TBS-everolimus is directly treated with HCl in methanol, avoiding intermediate isolation.
This method yields 39 g of crude this compound per 30 g sirolimus, with a final purity of >99% after HPLC.
Impurity Profiling and Process Improvements
A 2021 study identified dialkylated rapamycin derivatives (PG-D, PG-E, PG-F) as major byproducts during alkylation. By adjusting the base-to-triflate ratio and reaction time, researchers redirected selectivity toward PG-E, which is hydrolyzable to this compound. This optimization increased overall yield by 10% and reduced solvent consumption by 30%.
Table 3: Impact of Process Optimization
| Parameter | Traditional Method | Optimized Method |
|---|---|---|
| Yield (%) | 50–60 | 60–70 |
| Purity Before HPLC (%) | 60–65 | 75–80 |
| Reaction Time (h) | 12 | 6 |
Analytical Considerations in Synthesis
Quality control relies on HPLC and LC-MS to monitor intermediates and final product purity. For example, derivatization with N-methylaniline is used to quantify residual triflate intermediates. Regulatory submissions emphasize strict control over genotoxic impurities (e.g., triflic acid salts), requiring limits below 10 ppm .
Chemical Reactions Analysis
Types of Reactions: Everolimus undergoes various chemical reactions, including oxidation, reduction, and substitution. These reactions are essential for its metabolic processing and therapeutic action.
Common Reagents and Conditions:
Oxidation: Typically involves the use of oxidizing agents like hydrogen peroxide or potassium permanganate.
Reduction: Often carried out using reducing agents such as sodium borohydride or lithium aluminum hydride.
Substitution: Involves nucleophilic or electrophilic reagents under controlled conditions.
Major Products: The primary products formed from these reactions include various metabolites that are further processed in the body to exert their therapeutic effects .
Scientific Research Applications
Oncology Applications
Everolimus is primarily utilized in oncology for its antiproliferative properties. It has been approved by the United States Food and Drug Administration (FDA) for several cancer types, including:
- Renal Cell Carcinoma : this compound is indicated for advanced renal cell carcinoma after prior treatment with other therapies like sorafenib or sunitinib. In clinical trials, it demonstrated a median progression-free survival of 4.9 months compared to 1.9 months for placebo .
- Breast Cancer : In combination with letrozole, this compound has shown efficacy in treating hormone receptor-positive advanced breast cancer. A phase II trial revealed a notable improvement in progression-free survival .
- Gastric Cancer : A phase II study indicated that this compound could reduce the risk of disease progression in pretreated advanced gastric cancer patients, with a disease control rate significantly higher than placebo .
- Neuroendocrine Tumors : this compound is also approved for pancreatic neuroendocrine tumors, where it has shown a 65% decrease in tumor progression risk .
| Cancer Type | Indication | Median PFS (months) | Response Rate |
|---|---|---|---|
| Renal Cell Carcinoma | After prior therapy | 4.9 | 7% |
| Breast Cancer | With letrozole | Not specified | Not specified |
| Gastric Cancer | Advanced, previously treated | Not specified | 38.9% |
| Neuroendocrine Tumors | Advanced pancreatic neuroendocrine tumors | 12 | 65% decrease |
Transplantation Applications
This compound plays a crucial role in organ transplantation due to its immunosuppressive properties:
- Kidney Transplantation : It is used to prevent acute rejection in kidney transplant recipients, allowing for potential minimization or elimination of calcineurin inhibitors, which can have nephrotoxic effects .
- Other Organ Transplants : The drug has also been investigated for use in heart and liver transplants, showing promise in maintaining graft function and reducing rejection rates .
Other Therapeutic Uses
Beyond oncology and transplantation, this compound has been explored for various other conditions:
- Tuberous Sclerosis Complex : Approved for treating subependymal giant cell astrocytoma associated with tuberous sclerosis complex, this compound has shown efficacy in improving neurological function and survival rates in affected patients .
- Cardiovascular Applications : As a drug-eluting stent coating, this compound helps prevent restenosis by inhibiting smooth muscle cell proliferation .
Case Study 1: Advanced Renal Cell Carcinoma
A study involving patients with metastatic renal cell carcinoma treated with this compound showed an overall response rate of approximately 7%, with significant improvements in progression-free survival compared to placebo groups .
Case Study 2: Hormone Receptor-Positive Breast Cancer
In the MIRACLE trial, premenopausal women with advanced breast cancer receiving this compound plus letrozole exhibited enhanced outcomes compared to those on letrozole alone, demonstrating the potential of combination therapy in this setting .
Mechanism of Action
Everolimus exerts its effects by inhibiting the mTOR pathway, specifically targeting the mTORC1 protein complex. This inhibition disrupts cell growth, proliferation, and survival signals, leading to reduced tumor growth and immune response modulation. The molecular targets include various proteins involved in the PI3K/AKT/mTOR signaling pathway .
Comparison with Similar Compounds
Table 1: Structural and Pharmacokinetic Profiles of mTOR Inhibitors
| Compound | Structural Modification | Oral Bioavailability | Half-Life (h) | Key Pharmacokinetic Advantages |
|---|---|---|---|---|
| Everolimus | 2-hydroxyethyl chain at position 40 | ~30% | 30–40 | Improved solubility vs. sirolimus |
| Sirolimus | Unmodified macrolide | ~14% | 60–70 | Long half-life, but poor oral absorption |
| Temsirolimus | Esterified C40 hydroxyl group (prodrug) | N/A (IV only) | 17–24 | Water-soluble prodrug for intravenous use |
- Key Findings: this compound’s 2-hydroxyethyl substitution increases polarity, enhancing oral bioavailability compared to sirolimus . Temsirolimus, a prodrug, is administered intravenously due to poor oral absorption .
Efficacy in Specific Cancers
Pancreatic Neuroendocrine Tumors (pNETs)
Renal Cell Carcinoma (RCC)
- This compound vs. Nivolumab :
Mechanistic Differences
Table 2: Proteomic and Pathway Effects
- Key Findings :
Resistance and Biomarkers
- Cross-Resistance : this compound-resistant pNET cell lines (BON-1/R, QGP-1/R) show resistance to other rapalogs (e.g., temsirolimus) .
- Biomarkers: In breast cancer, PIK3CA mutations (e.g., H1047R) correlate with this compound efficacy (HR = 0.37 for PFS) . No consistent association between PI3K/AKT/mTOR pathway activity and response in biliary tract cancer .
Cost-Effectiveness
Table 3: Cost per Additional Month of Survival in RCC
| Treatment | Additional Cost/Month of OS vs. This compound |
|---|---|
| Cabozantinib | $48,773 |
| Nivolumab | $24,214 |
| Axitinib | Similar OS, lower cost |
- This compound remains cost-effective compared to axitinib but is less economical than nivolumab .
Biological Activity
Everolimus, a potent inhibitor of the mammalian target of rapamycin (mTOR), is an analog of rapamycin and has gained prominence in the treatment of various malignancies, including renal cell carcinoma and neuroendocrine tumors. This article delves into the biological activity of this compound, focusing on its mechanisms of action, pharmacodynamics, clinical efficacy, and safety profile, supported by data tables and relevant case studies.
This compound exerts its effects by binding to the FK506 binding protein-12 (FKBP-12), forming a complex that inhibits mTOR activity. This inhibition disrupts several downstream signaling pathways critical for cell growth and proliferation:
- Cell Cycle Regulation : By inhibiting mTOR, this compound blocks the progression of cells from the G1 phase to the S phase, leading to cell cycle arrest and apoptosis.
- Angiogenesis Inhibition : this compound reduces the expression of hypoxia-inducible factor (HIF) and vascular endothelial growth factor (VEGF), thereby inhibiting angiogenesis, which is crucial for tumor growth and metastasis .
- Metabolic Effects : The drug also impacts glucose uptake in cancer cells, further contributing to its anti-tumor effects .
Pharmacodynamics
The pharmacodynamic profile of this compound includes its effects on various biomarkers associated with mTOR signaling. A comprehensive study indicated that this compound effectively inhibited phosphorylation of ribosomal protein S6, a common marker for mTORC1 activity. The study also revealed that daily dosing at 10 mg provided more robust and prolonged inhibition compared to weekly dosing schedules .
Key Pharmacodynamic Findings
| Parameter | Daily Dosing (10 mg) | Weekly Dosing (50 mg) |
|---|---|---|
| S6 Phosphorylation Inhibition | Almost complete | Moderate |
| Proliferation Reduction | Significant | Moderate |
| Hyperphosphorylation of Akt | Observed in ~50% samples | Not maintained |
Clinical Efficacy
This compound has been evaluated in numerous clinical trials demonstrating its efficacy across various cancer types. Notably, it has shown significant activity in patients with renal cell carcinoma who are refractory to other therapies.
Case Studies
- Renal Cell Carcinoma : In a pivotal trial involving 410 patients with advanced renal cell carcinoma, this compound demonstrated a median progression-free survival (PFS) of 4.9 months compared to 1.9 months for placebo .
- Neuroendocrine Tumors : A study involving patients with advanced neuroendocrine tumors showed an overall response rate (ORR) of 32% when treated with this compound, highlighting its potential in this indication .
Safety Profile
While this compound is generally well-tolerated, it is associated with several adverse effects:
- Common Adverse Effects : Include stomatitis, infections (e.g., pneumonia), fatigue, and skin rash.
- Serious Adverse Events : Renal failure and proteinuria have been reported in clinical settings .
Table of Adverse Effects
| Adverse Effect | Incidence (%) |
|---|---|
| Stomatitis | 40 |
| Infections | 30 |
| Fatigue | 25 |
| Renal Failure | 10 |
Q & A
Q. How to design a crossover-adjusted OS analysis in this compound trials?
- Apply rank-preserving structural failure time models to account for placebo-to-everolimus crossover (85% in RADIANT-3). Sensitivity analyses (e.g., inverse probability weighting) mitigate confounding, though residual bias may persist .
Data Interpretation & Reporting
Q. Why do central vs. local PFS assessments differ in this compound trials?
Q. How to reconcile negative OS results despite significant PFS benefits?
- In BOLERO-2, crossover and subsequent therapies (e.g., CDK4/6 inhibitors) diluted OS signals. Pre-specified weighted analyses (e.g., RPSFTM) and post-hoc subgroup evaluations (e.g., biomarker-positive cohorts) clarify this compound' survival impact .
Emerging Research Directions
Q. What trial designs evaluate this compound in CDK4/6 inhibitor-resistant HR+ breast cancer?
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


