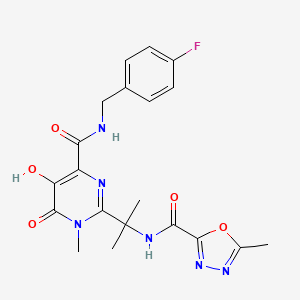
Raltegravir
Description
Historical Development and Discovery
Raltegravir emerged from systematic efforts to target HIV-1 integrase, a viral enzyme essential for inserting proviral DNA into host genomes. Early work in the 1990s identified β-diketo acids (DKAs) as inhibitors of integrase’s strand transfer activity, but their poor pharmacokinetic properties limited clinical utility. Merck researchers pivoted to structurally distinct scaffolds, leading to the discovery of 5,6-dihydroxypyrimidine-4-carboxamides and naphthyridine carboxamides as potent integrase inhibitors.
Key milestones include:
- 2000–2003 : Optimization of naphthyridine derivatives (e.g., L-870,810) demonstrated oral bioavailability and antiviral efficacy in animal models.
- 2004–2006 : Structural refinements introduced N-methyl-4-hydroxypyrimidinone-carboxamide moieties, enhancing metabolic stability. This yielded MK-0518 (this compound), which exhibited a 78 nM CIC95 in cell-based assays and favorable pharmacokinetics in preclinical species.
- 2007 : FDA approval of this compound marked the first integrase strand transfer inhibitor (INSTI) for HIV treatment, validated by phase III trials showing rapid viral load reduction.
Evolution from HCV Polymerase Inhibitors
The structural lineage of this compound traces to α,γ-diketo acids (DKAs) initially explored as hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase inhibitors. Key connections include:
| Feature | HCV NS5B Inhibitors | HIV Integrase Inhibitors |
|---|---|---|
| Core scaffold | Aryl diketo acids | Naphthyridine/dihydroxypyrimidine |
| Metal chelation | Mg2+/Zn2+ | Mg2+ in integrase active site |
| Pharmacophore | β-diketo motif | Oxadiazole/oxadiazolone substituents |
While DKAs like L-708,906 showed anti-HCV activity (IC50 = 45 nM), their repurposing for HIV integrase inhibition required scaffold hybridization. The 8-hydroxy-(1,6)-naphthyridine carboxamide in this compound retained critical metal-chelating distances (3.5–4.0 Å) but added π-stacking interactions via fluorobenzyl groups. This divergence minimized off-target effects on human polymerases.
Significance as First Approved Integrase Inhibitor
This compound’s 2007 approval revolutionized antiretroviral therapy by:
- Mechanistic novelty : Unlike reverse transcriptase/protease inhibitors, this compound blocks viral DNA integration via selective strand transfer inhibition (STI).
- Efficacy in resistant HIV : In phase III BENCHMRK trials, this compound + optimized background regimen achieved undetectable viral loads (<50 copies/mL) in 62% of multidrug-resistant patients vs. 33% with placebo.
- Distinct resistance profile : Primary mutations (Q148H/K/R, N155H) occur in integrase rather than overlapping with protease/reverse transcriptase mutations.
Comparative enzymatic studies revealed this compound’s 10-fold higher potency (IC50 = 2–7 nM) than early DKAs like S-1360 (IC50 = 200 nM). Its dissociation constant (Kd) of 15 nM for integrase-DNA complexes underscores target engagement specificity.
Chemical Classification and Structural Family
This compound (C20H21FN6O5) belongs to the N-alkylpyrimidinone carboxamide family, characterized by:
Key structural features :
- Metal-chelating core : 4-Oxadiazole and 5,6-dihydroxypyrimidin-4(1H)-one enable bidentate Mg2+ coordination.
- Hydrophobic substituents : 4-Fluorobenzyl and methyloxadiazole groups enhance binding to integrase’s hydrophobic pocket.
- Metabolic stability : N-methylation and oxadiazole ring reduce glucuronidation susceptibility vs. earlier DKAs.
Evolutionary comparison :
Properties
IUPAC Name |
N-[2-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxopyrimidin-2-yl]propan-2-yl]-5-methyl-1,3,4-oxadiazole-2-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CZFFBEXEKNGXKS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H21FN6O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2048660 | |
| Record name | Raltegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2048660 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
444.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
518048-05-0 | |
| Record name | Raltegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=518048-05-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Raltegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0518048050 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Raltegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06817 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Raltegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2048660 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.124.631 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | RALTEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/22VKV8053U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Raltegravir | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8124 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Preparation Methods
First-Generation Synthesis
The initial synthesis of raltegravir involved multi-step processes with inherent challenges, such as regioselectivity issues and impurity formation. Key steps included:
-
Formation of oxadiazole intermediates : Reaction of 2-amino-2-methylpropanenitrile with oxadiazole carbonyl chloride to form N-(2-cyanopropan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide (II).
-
Cyclization with dialkyl acetylene dicarboxylate (DMAD) : Treatment of hydroxylamine derivatives with DMAD under thermal conditions to construct the pyrimidinone core.
-
Amidation with p-fluorobenzylamine : Coupling of intermediates to introduce the 4-fluorobenzyl group.
-
Regioselective methylation : Use of trimethylsulfoxonium iodide to methylate the pyrimidinone nitrogen, avoiding O-methylation byproducts.
Table 1: Key Parameters of First-Generation Synthesis
*Overall yield from starting material.
Second-Generation Synthesis
Later optimizations addressed inefficiencies in prior routes, focusing on reduced steps and waste. A second-generation method employed:
-
Thermal rearrangement of amidoxime DMAD adducts : Direct cyclization to form the hydroxypyrimidinone core, eliminating protection/deprotection steps.
-
Highly selective methylation : Achieved >99% regioselectivity for N-methylation using optimized conditions.
Table 2: Advantages of Second-Generation Synthesis
| Parameter | First Generation | Second Generation |
|---|---|---|
| Overall Yield | 55–58% | 35% |
| Waste Reduction | N/A | 65% reduction |
| Methylation Selectivity | 90% | >99% |
| Steps | 9–10 | 9 |
Critical Process Innovations
Regioselective Methylation
Prior methods suffered from O-methylation side products, but optimized conditions using trimethylsulfoxonium iodide at 100°C achieved >90% yield with minimal byproducts. This eliminated the need for hydroxyl group protection, reducing time and cost.
Crystalline Form Control
The potassium salt of this compound (Form 3) was stabilized via:
Automated Radiolabeling
For pharmacokinetic studies, [¹⁸F]this compound was synthesized via:
-
Nucleophilic substitution : [¹⁸F]Fluoride ion reacted with 4-N,N,N-trimethylammoniumbenzonitrile triflate to form [¹⁸F]fluorobenzonitrile.
-
Reduction and coupling : Borohydride resin reduced the nitrile to [¹⁸F]fluorobenzylamine, which coupled with this compound precursors at 85°C.
Table 3: Automated Radiolabeling Efficiency
| Step | Yield (%) | Time (min) |
|---|---|---|
| [¹⁸F]FBA synthesis | 41 ± 6 | 135 total |
| Coupling to precursor | 4.61 ± 0.37 | 45 |
Comparison of Synthetic Routes
| Method | Steps | Overall Yield | Purity | Key Innovation |
|---|---|---|---|---|
| First Generation | 5–6 | 55–58% | >99.5% | Regioselective methylation |
| Second Generation | 9 | 35% | >99.5% | Thermal rearrangement, waste reduction |
| Radiolabeling | 2 | 4.61% | N/A | Automated [¹⁸F]FBA coupling |
Challenges and Solutions
-
Impurity Control : Prior methods generated E/Z isomers during DMAD cyclization. Optimized thermal conditions (110–140°C) minimized isomerization.
-
Cost and Scalability : The second-generation synthesis reduced organic/aqueous waste by 65% and improved productivity 3–4-fold.
-
Regulatory Compliance : All methods achieved this compound purity >99.5%, meeting stringent API standards .
Chemical Reactions Analysis
Synthetic Reactions of Raltegravir
The patented industrial synthesis of this compound involves a five-step route designed to minimize impurities and improve yield . Key reactions include:
Table 1: Key Synthetic Steps and Conditions
Key Improvements Over Prior Methods:
Stability and Degradation Reactions
This compound exhibits sensitivity to environmental and chemical factors, impacting its therapeutic efficacy:
Table 2: Degradation Pathways
Critical Stability Notes:
-
Polymorph screening identified anhydrous form A as the most stable crystalline phase .
-
Explosive dust-air mixtures may form during mechanical processing .
Reactivity with Functional Groups
This compound’s structure includes reactive moieties that influence its behavior:
-
Oxadiazole ring : Susceptible to nucleophilic attack under extreme pH .
-
Pyrimidinone core : Participates in methylation and tautomerization .
-
Amide bonds : Prone to hydrolysis in acidic/basic conditions .
Environmental and Metabolic Fate
Scientific Research Applications
Mechanism of Action
Raltegravir inhibits the catalytic activity of HIV integrase, preventing the integration of the viral genome into the human genome. This inhibition blocks the strand transfer step of HIV-1 integration, which is essential for viral replication. This compound is primarily metabolized by glucuronidation .
Comparison with Similar Compounds
Structural and Binding Mode Comparisons
Key INSTIs :
- Elvitegravir : Shares a diketo acid motif with raltegravir but requires pharmacokinetic boosting (e.g., with cobicistat). Both bind to the integrase active site via Mg²⁺ chelation. However, elvitegravir’s resistance profile overlaps partially with this compound, with cross-resistance observed in mutations like N155H and Q148H .
- Dolutegravir : A second-generation INSTI with a tricyclic carbamoyl pyridone structure. It binds more tightly to the integrase active site, retaining activity against some this compound-resistant mutants (e.g., Y143R, N155H) .
- Bictegravir : Structurally similar to dolutegravir but designed for once-daily dosing. It maintains efficacy against major this compound-resistant mutants (e.g., G140S/Q148H) .
Experimental Compounds :
Efficacy and Resistance Profiles
Pharmacokinetic and Pharmacodynamic Properties
Biological Activity
Raltegravir is an antiretroviral medication belonging to the class of integrase inhibitors, primarily used in the treatment of HIV-1 infection. This article explores its biological activity, mechanisms of action, efficacy in clinical settings, and safety profile based on diverse research findings.
This compound functions as an integrase strand transfer inhibitor (INSTI). It inhibits the integrase enzyme, which is crucial for the integration of viral DNA into the host cell genome. The drug binds to the integrase-DNA complex, preventing the strand transfer process essential for HIV replication. This binding is contingent upon the presence of divalent metal ions at the integrase active site, which are chelated by this compound's diketo group, forming a transient synaptic complex that halts integration .
Long-Term Efficacy
This compound has demonstrated significant efficacy in both treatment-naïve and treatment-experienced patients. In a comprehensive analysis of two pivotal phase III studies (BENCHMRK-1 and BENCHMRK-2), this compound was combined with optimized background therapy. At week 96, patients receiving this compound showed a viral load of less than 50 copies/mL in 51% of cases compared to 22% in the placebo group. The mean increase in CD4 cell count was 164 cells/µL for the this compound group versus 63 cells/µL for placebo .
Summary of Key Findings
| Study | Population | Treatment Group | Viral Load <50 copies/mL (%) | CD4 Cell Count Change (cells/µL) |
|---|---|---|---|---|
| BENCHMRK-1 & -2 | Treatment-experienced | This compound + OBT | 51% | +164 |
| BENCHMRK-1 & -2 | Treatment-experienced | Placebo + OBT | 22% | +63 |
Safety Profile
This compound has a favorable safety profile, with adverse events generally being mild and manageable. Common side effects include gastrointestinal disturbances and insomnia. Serious adverse events such as Stevens-Johnson syndrome and immune reconstitution inflammatory syndrome have been reported but are rare .
Adverse Events Overview
| Adverse Event | Incidence Rate (%) |
|---|---|
| Rash (including SJS) | <2% |
| Elevation in liver enzymes | Variable |
| Depression/Suicidal ideation | <2% |
Pharmacokinetics and Metabolism
This compound is primarily metabolized via glucuronidation, predominantly by UGT1A1. Studies indicate that about 70% of the administered dose is converted to its glucuronide metabolite. This compound does not significantly inhibit cytochrome P450 enzymes, suggesting a low potential for drug-drug interactions .
Case Studies
A real-world study assessed the effectiveness of this compound-based highly active antiretroviral therapy (HAART) in a cohort of patients starting treatment. The study found that after six months, 85% of patients achieved viral suppression (<1000 copies/mL), indicating robust efficacy similar to clinical trial outcomes .
Q & A
Q. What are the key pharmacokinetic considerations when studying Raltegravir in pregnant populations?
Pregnancy moderately impacts this compound’s free fraction but does not necessitate dose adjustments. Researchers should measure both free and glucuronidated this compound levels, as intersubject variability often exceeds pregnancy-related changes. Methodologically, population pharmacokinetic modeling can account for covariates like gestational age and protein binding shifts .
Q. How does this compound compare to efavirenz in treatment-naïve patients regarding safety and efficacy?
In the STARTMRK trial, this compound demonstrated non-inferior efficacy to efavirenz, with fewer drug-related adverse events (AEs), such as neuropsychiatric effects. Methodological insights: Use randomized controlled trial (RCT) designs with composite endpoints (e.g., viral suppression <50 copies/mL, CD4+ recovery) and adjust for baseline viral load/CD4+ strata .
Q. What is the evidence for this compound’s efficacy in treatment-experienced patients with multi-drug-resistant HIV?
The BENCHMRK trials showed superior virologic suppression (62.1% vs. 32.9% at 48 weeks) when this compound was added to optimized background therapy (OBT). Key methodological considerations: Use genotypic/phenotypic sensitivity scores to stratify patients and assess OBT potency .
Advanced Research Questions
Q. How do resistance-associated mutations (RAMs) to this compound emerge, and what methodologies capture quasispecies dynamics during virologic failure?
Resistance primarily occurs via two pathways: N155H or Q148K/R/H, often with secondary mutations (e.g., G140S). Advanced methods include clonal sequencing to detect minority variants and phenotypic assays to quantify integrase inhibitor susceptibility. Note: The 148 pathway confers higher resistance (50-fold vs. 10-fold for N155H) and is more stable .
Q. What methodological approaches resolve contradictions in long-term safety data, such as cancer risk or lipid abnormalities?
Meta-analyses of RCTs (e.g., STARTMRK/BENCHMRK) show no increased cancer risk (RR: 0.75–0.87 vs. comparators). For lipid profiles, pre-specified analyses adjusting for baseline dyslipidemia and concomitant medications are critical. Confounding factors (e.g., prior protease inhibitor use) require stratification .
Q. How can researchers design studies to evaluate this compound’s role in viral reservoir dynamics?
Utilize this compound intensification in virologically suppressed patients and measure 2-LTR circular DNA via droplet digital PCR. This approach distinguishes residual replication from latent reservoir activation. Longitudinal sampling (e.g., pre/post-intensification) controls for assay variability .
Q. What explains divergent efficacy outcomes in this compound-based second-line therapy across trials?
In the EARNEST trial, this compound did not outperform NRTIs when combined with protease inhibitors, contrasting with BENCHMRK. Key factors: Patient demographics (e.g., African vs. global cohorts), OBT composition (e.g., first-time use of darunavir/enfuvirtide), and adherence metrics. Sensitivity analyses should account for these variables .
Methodological Guidance for Data Analysis
Q. How should researchers handle missing data in long-term observational studies of this compound?
Use multiple imputation or inverse probability weighting to address attrition bias. For example, in Japanese cohort studies, exclusion criteria (e.g., missing CD4+/viral load data) must be transparently reported to avoid selection bias .
Q. What statistical models are optimal for analyzing time-to-event outcomes (e.g., virologic failure or adverse events)?
Cox proportional hazards models with time-varying covariates (e.g., adherence, drug interactions) are recommended. In STARTMRK, Kaplan-Meier curves showed cancer risk plateaued after 3 months, highlighting the need for time-stratified analyses .
Special Populations and Subgroup Analyses
Q. How does this compound perform in non-Western populations, such as Japanese cohorts?
Long-term data from Japan show sustained virologic suppression (92% at 7 years), comparable to global trials. Methodological note: Use Clopper-Pearson confidence intervals for proportions in small subgroups and adjust for regional ART prescribing patterns .
Q. What are the implications of sex/gender and race on this compound’s pharmacokinetics and efficacy?
Pooled analyses found no significant differences in efficacy by sex or race. However, covariates like body weight and CYP3A4 activity (influenced by genetics) require pharmacokinetic modeling with nonlinear mixed-effects (NLME) approaches .
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


