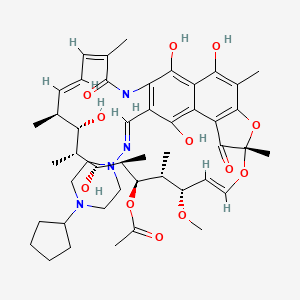
Rifapentine
Description
Systematic Nomenclature and Molecular Formula Analysis
The systematic nomenclature of rifapentine follows International Union of Pure and Applied Chemistry guidelines, reflecting its complex polycyclic structure with multiple functional groups and stereochemical centers. The complete systematic name is (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-26-[(E)-(4-cyclopentylpiperazin-1-yl)iminomethyl]-2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1^4,7.0^5,28]triaconta-1(29),2,4,9,19,21,25,27-octaen-13-yl acetate. This nomenclature systematically describes the stereochemical configuration at each asymmetric center, the geometric isomerism of double bonds, and the substitution pattern of the tetracyclic framework.
The molecular formula of this compound is C47H64N4O12, indicating a molecular composition of 47 carbon atoms, 64 hydrogen atoms, 4 nitrogen atoms, and 12 oxygen atoms. The molecular weight is calculated as 877.0307 atomic mass units, with the monoisotopic mass being 876.452073532. This substantial molecular weight reflects the complexity of the this compound structure, which incorporates multiple ring systems, hydroxyl groups, methyl substituents, and a characteristic piperazine moiety.
Alternative chemical names for this compound include rifamycin, 3-[[(4-cyclopentyl-1-piperazinyl)imino]methyl]-, 3-[(4-cyclopentyl-1-piperazinyl)iminomethyl]rifamycin, and cyclopentylrifampicin. These names highlight the key structural feature that distinguishes this compound from related rifamycin compounds: the presence of a cyclopentyl ring attached to the piperazine nitrogen. The Chemical Abstracts Service registry number for this compound is 61379-65-5, providing a unique identifier for database searches and regulatory documentation.
Properties
Key on ui mechanism of action |
Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. |
|---|---|
CAS No. |
61379-65-5 |
Molecular Formula |
C47H64N4O12 |
Molecular Weight |
877.0 g/mol |
IUPAC Name |
[(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-26-[(4-cyclopentylpiperazin-1-yl)iminomethyl]-2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.14,7.05,28]triaconta-1(29),2,4,9,19,21,25,27-octaen-13-yl] acetate |
InChI |
InChI=1S/C47H64N4O12/c1-24-13-12-14-25(2)46(59)49-37-32(23-48-51-20-18-50(19-21-51)31-15-10-11-16-31)41(56)34-35(42(37)57)40(55)29(6)44-36(34)45(58)47(8,63-44)61-22-17-33(60-9)26(3)43(62-30(7)52)28(5)39(54)27(4)38(24)53/h12-14,17,22-24,26-28,31,33,38-39,43,53-57H,10-11,15-16,18-21H2,1-9H3,(H,49,59)/b13-12+,22-17+,25-14-,48-23?/t24-,26+,27+,28+,33-,38-,39+,43+,47-/m0/s1 |
InChI Key |
WDZCUPBHRAEYDL-LYDPARFQSA-N |
SMILES |
CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)C |
Isomeric SMILES |
C[C@H]1/C=C/C=C(\C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)[C@](O4)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)/C |
Canonical SMILES |
CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCN(CC5)C6CCCC6)C |
Appearance |
Solid powder |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
>3 years if stored properly |
solubility |
Soluble in DMSO |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
Rifapentine; DL 473; DL-473; DL473; R 773; R-773; R773; |
Origin of Product |
United States |
Preparation Methods
Core Synthesis Pathway
The industrial synthesis of rifapentine follows a multi-step sequence beginning with rifamycin-S as the starting material. As detailed in Chinese patent CN103951677B, the process involves three principal stages: intermediate formation, hydrolysis, and condensation.
Intermediate Formation : Rifamycin-S reacts with dihydroxymethyl tert-butyl amine in solvents such as isopropyl acetate or ethyl acetate under controlled temperature conditions (40–60°C). This step produces the key intermediate, rifamycin oxazine, through nucleophilic substitution at the C3 position. The choice of solvent critically influences reaction efficiency, with ethyl acetate demonstrating superior oxidation resistance compared to tetrahydrofuran (THF), which historically caused aldehyde group oxidation to carboxylic acids.
Hydrolysis and Reduction : The rifamycin oxazine intermediate undergoes hydrolysis in methanol or ethanol using sodium bicarbonate or sodium bisulfite as catalysts. Reductive agents like ascorbic acid (0.5–2.0% w/w) are introduced to prevent oxidative degradation during this phase. Patent data indicate pH maintenance between 4.0–9.0 optimizes hydrolysis rates while minimizing byproduct formation.
Condensation with 1-Amino-4-cyclopentylpiperazine : The hydrolysate reacts with 1-amino-4-cyclopentylpiperazine in a polar aprotic solvent system. Thin-layer chromatography (TLC) monitoring ensures complete consumption of starting materials before proceeding to purification.
Crystallization and Purification Innovations
Multi-Stage Temperature-Controlled Crystallization
A breakthrough in this compound manufacturing involves sectional crystallization with precise temperature modulation (Figure 1). The CN103951677B patent describes a three-phase process:
Phase 1 (50–70°C) : Rapid addition of recrystallization solvent (ethanol:water, 3:1 v/v) induces primary nucleation. Crystal growth initiates within 30–45 minutes under continuous stirring (200–300 rpm).
Phase 2 (18–35°C) : Reduced solvent addition rate (50–100 L/h) allows spontaneous secondary nucleation. This gradual cooling prevents amorphous particle formation, achieving 85–92% crystalline yield.
Phase 3 (<20°C) : Final solvent addition at 10–50 L/h followed by batch-wise cooling (2–5°C/hour) produces uniform particles (D90 < 50 μm). Post-crystallization standing time exceeds 2 hours to ensure phase stability.
Advanced Washing Techniques
Post-crystallization purification employs a triphasic washing sequence:
-
Primary Wash : Ethyl acetate (3–5 bed volumes) removes residual solvents and low-polarity impurities
-
Secondary Wash : Cold methanol (0–4°C, 2–3 bed volumes) eliminates hydrophilic byproducts
-
Tertiary Wash : Ethanol-water (1:1 v/v) ensures final particle surface neutralization
This protocol reduces impurity content from initial 8–12% to <0.5% in final API batches.
Solvent and Catalyst Optimization
Solvent Selection Criteria
Comparative studies in patent CN103951677A demonstrate solvent impacts on reaction kinetics:
| Solvent | Reaction Rate (k, h⁻¹) | Byproduct Formation (%) |
|---|---|---|
| Ethyl acetate | 0.45 ± 0.03 | 1.2 ± 0.1 |
| THF | 0.38 ± 0.05 | 4.7 ± 0.3 |
| DMF | 0.51 ± 0.02 | 2.1 ± 0.2 |
| DMSO | 0.49 ± 0.04 | 3.8 ± 0.4 |
Ethyl acetate emerges as optimal, balancing reaction rate and purity.
Reductive Catalyst Systems
Quality Control and Analytical Validation
Recent pharmacological studies validate preparation methods through bioequivalence testing. Frontiers in Pharmacology research (2024) confirms this compound's linear pharmacokinetics (0.05–20.00 μg/mL) across production batches, reflecting consistency in crystallization and purification protocols. High-performance liquid chromatography (HPLC) profiles show inter-batch impurity variation <1.5%, meeting ICH Q3A guidelines.
Industrial Scale-Up Considerations
Reaction Engineering Parameters
-
Heat Transfer : Jacketed reactors maintain ±1°C accuracy during exothermic condensation
-
Mass Transfer : Impeller design (Rushton turbines) achieves mixing time <30 seconds
-
Filtration Efficiency : Centrifugal filters operate at 800–1200g for crystal recovery >95%
Chemical Reactions Analysis
Structural Basis for Reactivity
Rifapentine shares a core ansamycin structure with rifampin but incorporates a cyclopentyl ring substitution at the C3 position. This modification enhances its lipophilicity and influences interactions with bacterial RNA polymerase, where it binds the β-subunit to inhibit transcription . The molecule contains multiple reactive sites, including hydroxyl, amine, and quinone groups, which govern its degradation and metabolic pathways.
Table 1: Synthetic Pathway Optimization
| Parameter | Original Method | Improved Method (Patent CN111018886A) |
|---|---|---|
| Cyclization Environment | Alkaline | Acidic (pH 4–5) |
| Impurity Control | ≤1% | ≤0.1% |
| Solvent Residues | n-butanol: ≤2% | n-butanol: ≤0.5% |
Degradation Pathways
This compound undergoes hydrolysis and oxidative degradation:
-
Hydrolysis : The ester bond at C25 hydrolyzes to form 25-desacetyl this compound, a less active metabolite .
-
Maillard Reaction : Reacts with amino-containing excipients (e.g., isoniazid) under acidic conditions, producing isonicotinyl hydrazine derivatives .
-
Oxidation : Quinone groups in the ansa chain oxidize under high humidity or light, reducing potency .
Table 2: Stability Under Environmental Stressors
| Condition | Degradation Rate (24h) | Major Degradation Products |
|---|---|---|
| High Humidity (85% RH) | 12% | 25-desacetyl this compound |
| Acidic pH (pH 3) | 18% | Isonicotinyl hydrazine derivatives |
| Light Exposure (UV) | 15% | Oxidized ansa-chain byproducts |
Interaction with Co-Administered Drugs
This compound’s reactivity complicates combination therapies:
-
Isoniazid (INH) : Forms hydrazine adducts via nucleophilic substitution, requiring formulation separation to prevent interaction .
-
Pyrazinamide : No direct reaction, but this compound’s hepatic enzyme induction (4.5-fold vs. rifampin’s 3-fold) alters pyrazinamide metabolism .
Analytical Characterization
Key methods for monitoring reactions include:
-
Reverse-Phase HPLC : Resolves this compound from impurities (e.g., 25-desacetyl metabolite) with a C18 column and acetonitrile-phosphate buffer .
-
FTIR/NMR : Confirms structural integrity during synthesis, particularly cyclopentyl substitution .
Stabilization Strategies
-
Excipient Selection : Avoid amino acids and acidic stabilizers to prevent Maillard reactions .
-
Packaging : Use light-resistant, desiccant-containing containers to mitigate hydrolysis and oxidation .
This compound’s chemical profile underscores the need for precise synthesis and storage protocols to maintain efficacy. Ongoing research focuses on stabilizing its reactive sites while enhancing bioavailability through formulation innovations .
Scientific Research Applications
Treatment of Tuberculosis
Active Tuberculosis
Rifapentine is effective in treating drug-susceptible TB. It is often used in combination with other antitubercular agents such as isoniazid. The efficacy of this compound in short-course regimens has been demonstrated in clinical trials, showing comparable results to standard treatments while reducing treatment duration from six months to four months .
Latent Tuberculosis Infection
this compound has gained prominence in treating latent TB infection, particularly among high-risk populations. A regimen combining this compound and isoniazid (3HP) has been shown to be highly effective, allowing for a more manageable treatment course that enhances patient adherence . This combination therapy is especially beneficial for individuals with diabetes, who are at increased risk for progression to active TB .
Pharmacokinetics and Dosing Strategies
Recent studies have focused on the pharmacokinetics of this compound to optimize dosing strategies. Research indicates that weight-based dosing may not be appropriate for all patients, particularly those with lower body weights or those co-infected with HIV. Higher doses may be necessary to achieve therapeutic levels .
Table 1: Pharmacokinetic Characteristics of this compound
| Parameter | Value |
|---|---|
| Bioavailability | Decreases by 27% with HIV infection |
| Clearance Increase | Up to 72% after 21 days |
| Impact of Diet | Increased by 49% with high-fat meals |
| Recommended Dosing | Flat dosing suggested over weight-based |
Case Studies and Clinical Trials
Several clinical trials have explored the safety and efficacy of this compound in various populations:
- Study on Age Impact : A study examined how age affects treatment outcomes with this compound-based weekly therapy. Older patients exhibited different systemic drug reactions compared to younger cohorts, highlighting the need for age-specific treatment protocols .
- Combination Therapy : In trials assessing the combination of this compound with newer anti-TB drugs like SQ109, results indicated that while this compound was safe, its interaction with SQ109 did not enhance bacteriological outcomes significantly .
- Adverse Effects : A rare case of disseminated intravascular coagulation induced by rifampicin therapy was documented, underscoring the importance of monitoring patients for severe adverse effects during treatment .
Immunomodulatory Effects
Emerging research suggests that this compound may have immunomodulatory properties. It has been shown to influence immune pathways, potentially benefiting patients with inflammatory conditions . This aspect opens new avenues for research into its use beyond infectious diseases.
Mechanism of Action
Rifapentine exerts its effects by inhibiting DNA-dependent RNA polymerase in susceptible strains of Mycobacterium tuberculosis . This inhibition leads to the suppression of RNA synthesis, ultimately causing cell death . The molecular target of this compound is the β-subunit of the bacterial RNA polymerase .
Comparison with Similar Compounds
Comparison with Similar Compounds
Pharmacokinetic Properties
Rifapentine is compared to other rifamycins (rifampin, rifabutin) based on pharmacokinetic parameters:
| Parameter | This compound | Rifampin | Rifabutin |
|---|---|---|---|
| Half-life (t₁/₂) | 14–18 hours | 2–5 hours | 32–67 hours |
| Protein Binding | 97.7% | ≤88% | 85% |
| Cmax (600 mg dose) | ≤30 mg/L | ≤20 mg/L | ≤0.6 mg/L |
| Metabolites | 25-desacetyl-rifapentine (active) | Formyl derivatives (inactive) | Hydroxyl derivatives (inactive) |
| Enzyme Induction (CYP3A) | 85% potency of rifampin | Strong inducer | Moderate inducer |
- Half-life and Dosing : this compound’s longer half-life supports weekly dosing for LTBI, whereas rifampin requires daily administration. Rifabutin’s extended half-life is advantageous for Mycobacterium avium complex infections but less studied in TB .
- Protein Binding: this compound’s high protein binding (97.7%) reduces free drug availability, but free concentrations vary by ethnicity. African patients exhibit higher free this compound levels (0.091 µg/mL) despite lower total plasma concentrations compared to non-Africans (0.058 µg/mL), likely due to differences in albumin binding .
Antimicrobial Activity
- In Vitro Efficacy : this compound and rifabutin have comparable MICs against drug-susceptible M. tuberculosis (0.03–0.06 µg/mL for this compound; 0.015–0.06 µg/mL for rifabutin). However, this compound demonstrates superior bactericidal activity at equal concentrations .
- Metabolite Contribution : 25-desacetyl-rifapentine retains activity (MIC: 0.125–0.25 µg/mL), whereas rifampin’s metabolites are inactive .
Clinical Efficacy
- Latent TB : The 3HP regimen (12 weekly doses) achieved 85–96% efficacy, comparable to 9 months of daily isoniazid but with higher completion rates (89% vs. 64%) .
- Active TB : In a phase 2 trial, daily this compound (600 mg) showed 96% sputum culture conversion at 8 weeks, outperforming rifampin (94%) and demonstrating similar safety .
Cost and Accessibility
This compound is cost-prohibitive in high-burden countries due to single-supplier manufacturing. A 3HP regimen costs ~$80–$120 per patient, whereas 4 months of rifampin costs ~$20–$40 . Efforts to introduce generic versions are ongoing .
Key Research Findings
Pediatric Dosing : Children require 2.1-fold higher mg/kg doses than adults to achieve comparable AUCs due to faster clearance and lower bioavailability when tablets are crushed .
Ethnic Pharmacokinetics : African patients achieve free this compound concentrations twice the MIC of M. tuberculosis (0.091 µg/mL vs. 0.03–0.06 µg/mL) despite lower total drug levels, highlighting the role of protein binding variability .
Formulation Challenges : Crushed this compound tablets reduce bioavailability by 30%, necessitating improved pediatric formulations .
Q & A
Q. How should researchers analyze safety endpoints in trials combining this compound with moxifloxacin?
- Methodological Answer : Use competing risks regression to distinguish adverse events (AEs) leading to discontinuation from microbiological failures. Compare AE rates (e.g., grade ≥3 AEs) between arms via Fisher’s exact tests. Adjust for drug-drug interactions (e.g., this compound-induced moxifloxacin clearance) using PK-guided dose adjustments .
Contradiction Analysis
- Lower Efficacy in HIV Patients : Early trials reported reduced this compound exposure in HIV-positive patients due to decreased bioavailability . However, later PK models attribute this to unadjusted dosing and recommend 30% dose escalation, which improves efficacy parity .
- This compound vs. Rifampin : While this compound initially underperformed rifampin in weekly regimens , daily high-dose regimens (600–1200 mg) show comparable or superior efficacy when optimized for exposure .
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


