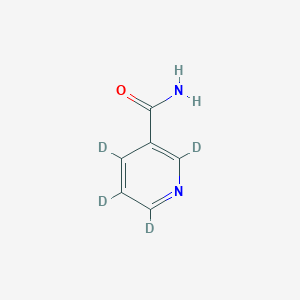
Nicotinamide-d4
Cat. No. B132304
Key on ui cas rn:
347841-88-7
M. Wt: 126.15 g/mol
InChI Key: DFPAKSUCGFBDDF-RHQRLBAQSA-N
Attention: For research use only. Not for human or veterinary use.
Patent
US06218543B1
Procedure details


In this Example a USP grade nicotinamide product was recovered from a crude nicotinamide product medium. The crude contained 39.7% nicotinamide, 2.15% sodium nicotinate and 0.14% 3-cyanopyridine. These and all other percentages given in this Example are percentages by weight unless indicated otherwise. The total sodium content of the medium was 0.38%. On a dry basis, the reaction crude contained 93.7% nicotinamide, 5.07% sodium nicotinate, 0.33% 3-cyanopyridine, and 0.9% total sodium. Cation and weak base resins were utilized in the recovery process. The cation exchange resin was Dowex Marathon C, a sulfonated copolymer of styrene and divinylbenzene (gel form). The weak base resin was Dowex Marathon WBA, a dimethylamine-functionalized chloromethylated copolymer of styrene and divinylbenzene (macroporous form with a monodisperse size distribution). After washing with deionized water, these resins were loaded into columns each having an inner diameter of 15 mm and a height of 30 cm, leaving about 1.5 inches head space at the top of the columns. The reaction crude was then successively treated over the cation-exchange resin (at 28 ml/min), the weak base resin (20 ml/min), the cation-exchange resin (28 ml/min), and the weak base resin (20 ml/min). The cation-exchange resin was regenerated after every ten bed volumes of reaction crude, by a cycle that included a water wash (20 ml/min, 1.25 bed volumes), a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes). The weak base resin was regenerated after every five bed volumes of reaction crude, by a cycle including a water wash (20 ml/min, 1.6 bed volumes), a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes). The feeds were analyzed by HPLC after the first pass cation-exchange and weak base, and second pass cation-exchange and weak base. Typical results from such experiments are presented in Table 1 below, top. The lower section of Table 1 gives a typical product analysis on a water free basis. The extreme right hand column of Table 1 sets out the results of an analysis of the product after recovery by evaporation (no crystallization performed). As can be seen, this processing reduced the 0.9% initial sodium to an undetectable level, and the initial 5.07% nicotinate to 0.13% (as nicotinic acid) on a dry weight basis, providing a USP grade nicotinamide.


















Identifiers


|
REACTION_CXSMILES
|
[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1.[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[Na+].C(C1C=NC=CC=1)#N.[Na].C=CC1C=CC=CC=1.C(C1C=CC=CC=1C=C)=C.CNC>O>[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:10]([OH:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1 |f:1.2,^1:27|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CNC
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Eight
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Nine
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Ten
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Eleven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Twelve
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Thirteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fourteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fifteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step 16
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step 17
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step 18
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
On a dry basis, the reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
After washing with deionized water
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
a height of 30 cm, leaving about 1.5 inches
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction crude
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was then successively treated over the cation-exchange resin (at 28 ml/min)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every ten bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.25 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every five bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.6 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Typical results from such experiments
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
results of an analysis of the product after recovery
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
by evaporation (
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
no crystallization
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-]
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 5.07% |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06218543B1
Procedure details


In this Example a USP grade nicotinamide product was recovered from a crude nicotinamide product medium. The crude contained 39.7% nicotinamide, 2.15% sodium nicotinate and 0.14% 3-cyanopyridine. These and all other percentages given in this Example are percentages by weight unless indicated otherwise. The total sodium content of the medium was 0.38%. On a dry basis, the reaction crude contained 93.7% nicotinamide, 5.07% sodium nicotinate, 0.33% 3-cyanopyridine, and 0.9% total sodium. Cation and weak base resins were utilized in the recovery process. The cation exchange resin was Dowex Marathon C, a sulfonated copolymer of styrene and divinylbenzene (gel form). The weak base resin was Dowex Marathon WBA, a dimethylamine-functionalized chloromethylated copolymer of styrene and divinylbenzene (macroporous form with a monodisperse size distribution). After washing with deionized water, these resins were loaded into columns each having an inner diameter of 15 mm and a height of 30 cm, leaving about 1.5 inches head space at the top of the columns. The reaction crude was then successively treated over the cation-exchange resin (at 28 ml/min), the weak base resin (20 ml/min), the cation-exchange resin (28 ml/min), and the weak base resin (20 ml/min). The cation-exchange resin was regenerated after every ten bed volumes of reaction crude, by a cycle that included a water wash (20 ml/min, 1.25 bed volumes), a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes). The weak base resin was regenerated after every five bed volumes of reaction crude, by a cycle including a water wash (20 ml/min, 1.6 bed volumes), a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes). The feeds were analyzed by HPLC after the first pass cation-exchange and weak base, and second pass cation-exchange and weak base. Typical results from such experiments are presented in Table 1 below, top. The lower section of Table 1 gives a typical product analysis on a water free basis. The extreme right hand column of Table 1 sets out the results of an analysis of the product after recovery by evaporation (no crystallization performed). As can be seen, this processing reduced the 0.9% initial sodium to an undetectable level, and the initial 5.07% nicotinate to 0.13% (as nicotinic acid) on a dry weight basis, providing a USP grade nicotinamide.


















Identifiers


|
REACTION_CXSMILES
|
[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1.[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[Na+].C(C1C=NC=CC=1)#N.[Na].C=CC1C=CC=CC=1.C(C1C=CC=CC=1C=C)=C.CNC>O>[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:10]([OH:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1 |f:1.2,^1:27|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CNC
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Eight
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Nine
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Ten
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Eleven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Twelve
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Thirteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fourteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fifteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step 16
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step 17
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step 18
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
On a dry basis, the reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
After washing with deionized water
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
a height of 30 cm, leaving about 1.5 inches
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction crude
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was then successively treated over the cation-exchange resin (at 28 ml/min)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every ten bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.25 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every five bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.6 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Typical results from such experiments
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
results of an analysis of the product after recovery
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
by evaporation (
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
no crystallization
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-]
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 5.07% |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06218543B1
Procedure details


In this Example a USP grade nicotinamide product was recovered from a crude nicotinamide product medium. The crude contained 39.7% nicotinamide, 2.15% sodium nicotinate and 0.14% 3-cyanopyridine. These and all other percentages given in this Example are percentages by weight unless indicated otherwise. The total sodium content of the medium was 0.38%. On a dry basis, the reaction crude contained 93.7% nicotinamide, 5.07% sodium nicotinate, 0.33% 3-cyanopyridine, and 0.9% total sodium. Cation and weak base resins were utilized in the recovery process. The cation exchange resin was Dowex Marathon C, a sulfonated copolymer of styrene and divinylbenzene (gel form). The weak base resin was Dowex Marathon WBA, a dimethylamine-functionalized chloromethylated copolymer of styrene and divinylbenzene (macroporous form with a monodisperse size distribution). After washing with deionized water, these resins were loaded into columns each having an inner diameter of 15 mm and a height of 30 cm, leaving about 1.5 inches head space at the top of the columns. The reaction crude was then successively treated over the cation-exchange resin (at 28 ml/min), the weak base resin (20 ml/min), the cation-exchange resin (28 ml/min), and the weak base resin (20 ml/min). The cation-exchange resin was regenerated after every ten bed volumes of reaction crude, by a cycle that included a water wash (20 ml/min, 1.25 bed volumes), a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes). The weak base resin was regenerated after every five bed volumes of reaction crude, by a cycle including a water wash (20 ml/min, 1.6 bed volumes), a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes). The feeds were analyzed by HPLC after the first pass cation-exchange and weak base, and second pass cation-exchange and weak base. Typical results from such experiments are presented in Table 1 below, top. The lower section of Table 1 gives a typical product analysis on a water free basis. The extreme right hand column of Table 1 sets out the results of an analysis of the product after recovery by evaporation (no crystallization performed). As can be seen, this processing reduced the 0.9% initial sodium to an undetectable level, and the initial 5.07% nicotinate to 0.13% (as nicotinic acid) on a dry weight basis, providing a USP grade nicotinamide.


















Identifiers


|
REACTION_CXSMILES
|
[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1.[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[Na+].C(C1C=NC=CC=1)#N.[Na].C=CC1C=CC=CC=1.C(C1C=CC=CC=1C=C)=C.CNC>O>[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:10]([OH:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1 |f:1.2,^1:27|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CNC
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Eight
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Nine
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Ten
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Eleven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Twelve
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Thirteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fourteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fifteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step 16
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step 17
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step 18
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
On a dry basis, the reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
After washing with deionized water
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
a height of 30 cm, leaving about 1.5 inches
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction crude
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was then successively treated over the cation-exchange resin (at 28 ml/min)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every ten bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.25 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every five bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.6 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Typical results from such experiments
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
results of an analysis of the product after recovery
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
by evaporation (
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
no crystallization
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-]
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 5.07% |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US06218543B1
Procedure details


In this Example a USP grade nicotinamide product was recovered from a crude nicotinamide product medium. The crude contained 39.7% nicotinamide, 2.15% sodium nicotinate and 0.14% 3-cyanopyridine. These and all other percentages given in this Example are percentages by weight unless indicated otherwise. The total sodium content of the medium was 0.38%. On a dry basis, the reaction crude contained 93.7% nicotinamide, 5.07% sodium nicotinate, 0.33% 3-cyanopyridine, and 0.9% total sodium. Cation and weak base resins were utilized in the recovery process. The cation exchange resin was Dowex Marathon C, a sulfonated copolymer of styrene and divinylbenzene (gel form). The weak base resin was Dowex Marathon WBA, a dimethylamine-functionalized chloromethylated copolymer of styrene and divinylbenzene (macroporous form with a monodisperse size distribution). After washing with deionized water, these resins were loaded into columns each having an inner diameter of 15 mm and a height of 30 cm, leaving about 1.5 inches head space at the top of the columns. The reaction crude was then successively treated over the cation-exchange resin (at 28 ml/min), the weak base resin (20 ml/min), the cation-exchange resin (28 ml/min), and the weak base resin (20 ml/min). The cation-exchange resin was regenerated after every ten bed volumes of reaction crude, by a cycle that included a water wash (20 ml/min, 1.25 bed volumes), a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes). The weak base resin was regenerated after every five bed volumes of reaction crude, by a cycle including a water wash (20 ml/min, 1.6 bed volumes), a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes). The feeds were analyzed by HPLC after the first pass cation-exchange and weak base, and second pass cation-exchange and weak base. Typical results from such experiments are presented in Table 1 below, top. The lower section of Table 1 gives a typical product analysis on a water free basis. The extreme right hand column of Table 1 sets out the results of an analysis of the product after recovery by evaporation (no crystallization performed). As can be seen, this processing reduced the 0.9% initial sodium to an undetectable level, and the initial 5.07% nicotinate to 0.13% (as nicotinic acid) on a dry weight basis, providing a USP grade nicotinamide.


















Identifiers


|
REACTION_CXSMILES
|
[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1.[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[Na+].C(C1C=NC=CC=1)#N.[Na].C=CC1C=CC=CC=1.C(C1C=CC=CC=1C=C)=C.CNC>O>[C:10]([O-:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:10]([OH:18])(=[O:17])[C:11]1[CH:16]=[CH:15][CH:14]=[N:13][CH:12]=1.[C:1]([NH2:9])(=[O:8])[C:2]1[CH:7]=[CH:6][CH:5]=[N:4][CH:3]=1 |f:1.2,^1:27|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Six
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CNC
|
Step Seven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C=CC1=CC=CC=C1
|
Step Eight
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(=C)C1=C(C=CC=C1)C=C
|
Step Nine
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Ten
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Eleven
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Twelve
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Thirteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fourteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Step Fifteen
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-].[Na+]
|
Step 16
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(#N)C=1C=NC=CC1
|
Step 17
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[Na]
|
Step 18
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
On a dry basis, the reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
After washing with deionized water
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
a height of 30 cm, leaving about 1.5 inches
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
The reaction crude
|
ADDITION
|
Type
|
ADDITION
|
|
Details
|
was then successively treated over the cation-exchange resin (at 28 ml/min)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every ten bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.25 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 12% sulfuric acid strip (7 ml/min, 1 bed volume), and another water wash (20 ml/min, 1.25 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
after every five bed volumes of reaction crude
|
WASH
|
Type
|
WASH
|
|
Details
|
wash (20 ml/min, 1.6 bed volumes)
|
WASH
|
Type
|
WASH
|
|
Details
|
a 4% sodium hydroxide strip (20 ml/min, 1 bed volume), and another water wash (2.6 bed volumes)
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
Typical results from such experiments
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
results of an analysis of the product after recovery
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
by evaporation (
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
no crystallization
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)[O-]
|
Measurements
| Type | Value | Analysis |
|---|---|---|
| YIELD: PERCENTYIELD | 5.07% |
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)O
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C1=CN=CC=C1)(=O)N
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
