Mebendazole
Descripción general
Descripción
Mebendazole is a benzimidazole-class anthelmintic drug first introduced in the 1970s. It exerts its primary therapeutic effects by binding to the colchicine-binding site (CBS) of β-tubulin, inhibiting microtubule polymerization, and disrupting cellular processes such as mitosis and nutrient absorption in parasites . Beyond its antiparasitic applications, this compound has shown promise in oncology, virology, and immunomodulation. Preclinical studies highlight its ability to inhibit cancer cell proliferation, induce apoptosis, and suppress angiogenesis . Recent clinical trials also demonstrate its efficacy in reducing inflammation and enhancing innate immunity in COVID-19 patients .
Métodos De Preparación
Synthetic Routes for Mebendazole Production
Condensation and Cyclization via 3,4-Diaminobenzophenone
The most widely documented method for synthesizing this compound begins with 3,4-diaminobenzophenone as a key intermediate. This compound reacts with methyl cyanocarbamate in a cyclization reaction facilitated by acidic conditions.
Procedure :
- Reaction Setup : 3,4-Diaminobenzophenone is dissolved in acetone and treated with hydrochloric acid to protonate the amine groups.
- Cyclization : Methyl cyanocarbamate is introduced, initiating a nucleophilic attack by the primary amine on the carbonyl group of the carbamate. This forms the benzimidazole core structure.
- Salt Formation : The crude product is treated with nitric acid in methanol to yield this compound nitrate, which enhances crystallinity.
- Crystallization : Conversion to the thermodynamically stable C-polymorph is achieved through controlled cooling and seeding.
Key Parameters :
- Temperature: 35–45°C during cyclization.
- Solvent System: Acetone for initial reaction; methanol for salt formation.
- Yield: 90% for crude product, 95% after crystallization.
Alternative Synthesis from o-Dichlorobenzene and Benzoyl Chloride
An alternative route starts with o-dichlorobenzene and benzoyl chloride, avoiding the need for pre-synthesized 3,4-diaminobenzophenone.
Procedure :
- Condensation : o-Dichlorobenzene reacts with benzoyl chloride in the presence of aluminum chloride to form 3,4-dichlorobenzophenone.
- Ammoniation : The dichloro intermediate undergoes ammonolysis with aqueous ammonia, replacing chlorine atoms with amine groups.
- Cyclization : The resulting 3,4-diaminobenzophenone is treated with methyl cyanocarbamate under reflux in formic acid to complete the benzimidazole ring.
Key Parameters :
- Catalyst: Aluminum chloride for Friedel-Crafts acylation.
- Reaction Time: 6–7 hours at 80°C during cyclization.
- Yield: 85–90% for the final product.
Optimization of Reaction Conditions
Solvent and Catalytic Systems
The choice of solvent significantly impacts reaction efficiency. Acetone and formic acid are preferred for their ability to stabilize intermediates and facilitate proton transfer. Catalytic amounts of hydrochloric acid accelerate cyclization by activating the carbonyl group of methyl cyanocarbamate.
Comparative Data :
| Parameter | Acetone/HCl System | Formic Acid Reflux |
|---|---|---|
| Temperature (°C) | 40 | 80 |
| Reaction Time (hours) | 16 | 6 |
| Crude Yield (%) | 90 | 85 |
| Final Purity (%) | 95 | 95 |
Polymorph Control
This compound exists in multiple polymorphic forms, with the C-crystal being the most therapeutically stable. Seeding with C-polymorph crystals during cooling ensures uniform crystallization. Methanol and water mixtures (3:1 v/v) are optimal for inducing nucleation.
Purification and Crystallization Techniques
Salt Formation
Conversion to this compound nitrate improves solubility for subsequent purification. The nitrate salt is precipitated by adding methanol to the reaction mixture, achieving 98% purity after filtration.
Recrystallization
Crude this compound is dissolved in hot formic acid (65°C) and treated with activated carbon to adsorb impurities. Slow addition of water induces crystallization, yielding needle-like C-polymorph crystals.
Conditions :
- Solvent: Formic acid/water (4:1 ratio).
- Cooling Rate: 1°C per minute to prevent amorphous phase formation.
Analytical Methods for Quality Control
Spectrophotometric Analysis
A validated UV-Vis method quantifies this compound using oxidative coupling with N-bromosuccinimide (NBS) and Rhodamine B. The absorbance at 570 nm correlates linearly with concentration (2.5–30 μg/mL, R² = 0.9992).
Procedure :
- Oxidation : this compound is treated with excess NBS in 0.1 M HCl.
- Coupling : The oxidized product reacts with Rhodamine B, forming a chromophore.
- Detection : Absorbance measured at λ_max = 570 nm.
High-Performance Liquid Chromatography (HPLC)
Reverse-phase HPLC with C18 columns and acetonitrile/water mobile phases (70:30 v/v) resolves this compound from degradation products. Retention time: 6.2 minutes.
Comparative Analysis of Preparation Methods
Análisis De Reacciones Químicas
Tipos de reacciones
El mebendazol experimenta diversas reacciones químicas, incluyendo reacciones de oxidación, reducción y sustitución.
Reactivos y condiciones comunes
Oxidación: Se pueden utilizar oxidantes comunes como el peróxido de hidrógeno o el permanganato de potasio.
Reducción: Se pueden emplear agentes reductores como el catalizador de paladio sobre carbón en presencia de hidrógeno.
Sustitución: Las reacciones de sustitución suelen implicar el uso de agentes halogenantes o nucleófilos.
Productos principales
Los productos principales que se forman a partir de estas reacciones dependen de los reactivos y las condiciones específicas que se utilicen. Por ejemplo, la reducción de la 4-amino-3-nitrobenzofenona con catalizador de paladio sobre carbón produce 3,4-diaminobenzofenona .
Aplicaciones Científicas De Investigación
El mebendazol se ha reutilizado para diversas aplicaciones de investigación científica más allá de su uso original como antihelmíntico. Ha mostrado promesa en el tratamiento de cánceres cerebrales, incluyendo el glioma, al inhibir la progresión maligna y aumentar la sensibilidad de las células gliomatosas a la quimioterapia o radioterapia convencionales . Además, se ha explorado el mebendazol por sus propiedades anticancerígenas en múltiples cánceres, incluyendo la leucemia mieloide aguda, el cáncer de mama y el cáncer gastrointestinal .
Mecanismo De Acción
El mebendazol funciona inhibiendo la polimerización de la tubulina en los parásitos, interrumpiendo la formación de microtúbulos e interfiriendo con la absorción de glucosa . Esto conduce en última instancia a la muerte del parásito. El compuesto se dirige a vías críticas implicadas en la proliferación celular, la apoptosis y la invasión/migración .
Comparación Con Compuestos Similares
Structural Similarities and Differences
Mebendazole shares a benzimidazole core structure with other compounds in its class, including albendazole , flubendazole , oxibendazole , and parbendazole . Structural variations occur at the substituent groups:
- Flubendazole : A fluoroanalog of this compound, differing by a fluorine substitution at the para position of the benzene ring .
- Albendazole : Features a propylthio group at the carbamate side chain, enhancing lipophilicity and bioavailability .
- Oxibendazole : Contains an oxy group in place of the methyl group in this compound, altering solubility and pharmacokinetics .
Mechanism of Action and Tubulin Binding Affinity
All benzimidazoles target β-tubulin, but binding affinities vary across isoforms and species. Studies comparing this compound, nocodazole, and albendazole reveal:
| Compound | Kd (BTb) | Kd (CeTb) | Selectivity Ratio (BTb/CeTb) |
|---|---|---|---|
| This compound | ~1 µM | ~1 µM | 1.0 |
| Nocodazole | ~1 µM | ~2.5 µM | 0.4 |
| Albendazole | ND* | Moderate | Not detected in BTb |
ND = Not Determined
This compound and nocodazole exhibit comparable binding to both blood cell-specific (BTb) and Caenorhabditis elegans (CeTb) tubulin isoforms, while albendazole shows negligible affinity for BTb .
Anticancer Activity
This compound outperforms several analogs in preclinical cancer models:
Table 3: Anticancer Efficacy in Human Cell Lines
| Compound | IC50 (KT21MG1 meningioma) | Caspase-3/7 Activation (Fold vs. Control) |
|---|---|---|
| This compound | 0.5–1 µM | 3.5–4.2 (dose-dependent) |
| Flubendazole | 0.8–1.2 µM | 2.8–3.1 |
| Oxibendazole | >10 µM | No significant effect |
This compound induces mitochondrial cytochrome c release and apoptosis in lung cancer cells, while flubendazole shows comparable efficacy in neuroblastoma models .
Antiviral and Immunomodulatory Effects
- COVID-19 : Reduces C-reactive protein (CRP) by 40% and increases cycle threshold (CT) values in 3 days, indicating viral load reduction .
- Comparative Data : In silico studies identify this compound, oxibendazole, and albendazole as SARS-CoV-2 inhibitors, but only this compound has Phase II clinical trial support .
Pharmacokinetics and Solubility
| Compound | Bioavailability | Solubility Enhancers |
|---|---|---|
| This compound | 2–10% | Sodium salicylate, hydrotropes |
| Albendazole | ~30% | Novel salts (e.g., mesylate) |
| Flubendazole | <5% | Limited data |
This compound’s solubility improves with hydrotropes like sodium salicylate (up to 3 mol/L), but its bioavailability remains lower than albendazole’s .
Clinical Trial Data
- Oncology: this compound reduces tumor xenograft growth by 70% in mice and enhances radiation sensitivity in meningioma .
- COVID-19 : Phase II trials show faster inflammation resolution vs. placebo (p < 0.05) .
- Parasitic Diseases: Albendazole demonstrates superior cure rates (90% vs. 75%) in trichinosis compared to this compound .
Actividad Biológica
Mebendazole is a broad-spectrum anthelmintic drug primarily used to treat parasitic infections. However, recent studies have unveiled its potential in oncology, particularly its biological activity against various cancer types. This article delves into the biological mechanisms, pharmacokinetics, and clinical applications of this compound, supported by data tables and case studies.
Antiparasitic Activity
this compound works by inhibiting the polymerization of tubulin, which is essential for microtubule formation in parasites. This disruption leads to impaired glucose uptake and energy depletion in the parasites, ultimately resulting in their death.
Antitumor Activity
Recent research highlights this compound's potential as an anticancer agent. It has been shown to induce apoptosis in cancer cells through several mechanisms:
- Bcl-2 Inactivation : this compound induces apoptosis in melanoma cells by phosphorylating Bcl-2, which prevents its interaction with the pro-apoptotic protein Bax, thus promoting cell death .
- Cell Cycle Arrest : It has been observed to cause cell cycle arrest in various cancer cell lines, including ovarian and colorectal cancers .
- Inhibition of Tumor Angiogenesis : this compound reduces angiogenesis by inhibiting VEGFR2 kinase activity, leading to decreased microvessel density in tumors .
Table 1: Biological Activities of this compound
| Activity | Mechanism | Cancer Type |
|---|---|---|
| Apoptosis induction | Bcl-2 phosphorylation | Melanoma |
| Cell cycle arrest | Inhibition of tubulin polymerization | Ovarian cancer |
| Angiogenesis inhibition | VEGFR2 kinase inhibition | Colorectal cancer |
Pharmacokinetics
This compound exhibits variable pharmacokinetic properties influenced by dosage and formulation. Studies indicate that plasma levels increase with higher doses, and the drug achieves a maximum concentration within hours post-administration.
Table 2: Pharmacokinetic Parameters of this compound
| Parameter | Value |
|---|---|
| Bioavailability | Approximately 50% |
| Peak plasma concentration | 590 nM (after standard dosing) |
| Half-life | 3-6 hours |
Case Study: this compound in Glioblastoma Treatment
A phase II clinical trial investigated the combination of this compound with temozolomide in patients with newly diagnosed high-grade glioma. The study enrolled 24 patients, revealing promising results regarding safety and overall survival:
- Median Overall Survival (OS) : 21 months.
- Progression-Free Survival (PFS) : 13.1 months for patients receiving more than one month of treatment .
Table 3: Clinical Outcomes from Glioblastoma Study
| Outcome Measure | Result |
|---|---|
| Median OS | 21 months |
| Median PFS | 13.1 months |
| Adverse Events | Elevated ALT/AST at higher doses |
Case Study: this compound for COVID-19
A recent randomized controlled trial indicated that this compound therapy improved innate immunity and reduced inflammation markers in COVID-19 outpatients compared to a placebo group. Significant reductions in C-reactive protein (CRP) levels were noted within three days of treatment .
Q & A
Basic Research Question: What experimental design considerations are critical for evaluating mebendazole’s pharmacokinetics in heterogeneous populations?
Methodological Answer:
To assess pharmacokinetics (PK) in diverse populations, researchers should:
- Define subpopulations (e.g., neonates, immunocompromised individuals) based on metabolic or physiological differences .
- Use population PK modeling to account for variability in drug absorption, distribution, and clearance. For example, sparse sampling in pediatric cohorts can reduce ethical and logistical challenges .
- Validate assays (e.g., HPLC, differential pulse polarography) to ensure sensitivity in detecting low plasma concentrations .
- Data sharing protocols must comply with ethical standards, including anonymization and controlled access to sensitive datasets .
Advanced Research Question: How can conflicting efficacy data for this compound in repurposed oncology studies be reconciled?
Methodological Answer:
Conflicting results often arise from:
- Variability in experimental models : Compare outcomes across cell lines (e.g., LNCaP vs. DU145 prostate cancer cells) and in vivo models (e.g., xenografts vs. genetically engineered mice) .
- Dose-response discordance : Use dose-ranging studies to identify therapeutic thresholds. For example, this compound’s anti-cancer effects in PDE4D7-knockdown LNCaPs occur at lower doses than in wild-type cells .
- Mechanistic heterogeneity : Conduct transcriptomic or proteomic analyses to map pathways (e.g., cAMP dynamics, microtubule disruption) influenced by tumor microenvironment factors .
- Meta-analysis frameworks : Apply PRISMA guidelines to aggregate preclinical data and identify bias sources (e.g., publication bias, model selection) .
Basic Research Question: What validated analytical methods are recommended for quantifying this compound in pharmaceutical formulations?
Methodological Answer:
- Electrochemical techniques : Differential pulse polarography (DPP) offers sensitivity at µg/mL levels, validated in pH 7.4 buffers to mimic physiological conditions .
- Chromatography : HPLC with UV detection (λ = 254 nm) provides reproducibility, but requires column optimization to separate this compound from excipients .
- Quality control : Cross-validate results with mass spectrometry (LC-MS) to confirm specificity, especially in complex matrices like serum .
Advanced Research Question: What strategies address this compound’s solubility limitations in preclinical testing?
Methodological Answer:
- Co-solvent systems : Test biocompatible solvents (e.g., PEG-400) to enhance aqueous solubility while monitoring cytotoxicity in vitro .
- Nanoformulation : Develop liposomal or polymeric nanoparticles to improve bioavailability. Characterize particle size (DLS) and encapsulation efficiency (UV-Vis) .
- In silico modeling : Use tools like COSMO-RS to predict solubility in simulated biological fluids and guide formulation design .
Basic Research Question: How should researchers design studies to evaluate this compound resistance in helminthic parasites?
Methodological Answer:
- Longitudinal sampling : Collect parasite isolates pre- and post-treatment to track β-tubulin mutations linked to resistance .
- Phenotypic assays : Measure IC50 shifts in larval motility or egg hatching inhibition assays across multiple generations .
- Genomic sequencing : Identify SNPs in β-tubulin isotype-1 genes and correlate with clinical failure rates .
Advanced Research Question: What methodologies optimize this compound combination therapies for synergistic anti-helminthic effects?
Methodological Answer:
- Checkerboard assays : Determine fractional inhibitory concentration indices (FICI) for this compound paired with albendazole or ivermectin .
- Mechanistic synergy : Use RNAi or CRISPR to validate target pathways (e.g., dual β-tubulin and glutamate-gated chloride channel disruption) .
- In vivo validation : Employ factorial design experiments in rodent models to assess efficacy-toxicity trade-offs .
Basic Research Question: How to ensure reproducibility in this compound’s in vitro cytotoxicity assays?
Methodological Answer:
- Standardize cell lines : Use authenticated stocks (e.g., ATCC-certified DU145) and control for passage number .
- Culture conditions : Maintain consistent O2 levels (5% CO2) and serum concentrations (10% FBS) to minimize batch effects .
- Endpoint validation : Combine MTT assays with live-cell imaging to confirm apoptosis vs. necrosis .
Advanced Research Question: How can computational models predict this compound’s off-target effects in repurposing studies?
Methodological Answer:
- Docking simulations : Use AutoDock Vina to screen this compound against human kinases or GPCRs implicated in side effects .
- Network pharmacology : Construct protein-protein interaction networks to identify secondary targets (e.g., PDE4D7 in prostate cancer) .
- Toxicogenomics : Apply LINCS database queries to predict gene expression changes in non-target tissues .
Propiedades
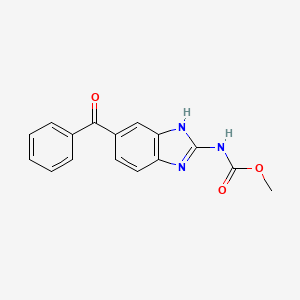
IUPAC Name |
methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H13N3O3/c1-22-16(21)19-15-17-12-8-7-11(9-13(12)18-15)14(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,17,18,19,21) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OPXLLQIJSORQAM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H13N3O3 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4040682 | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
295.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Mebendazole is a white to slightly yellow powder. Pleasant taste. Practically water insoluble. (NTP, 1992), Solid | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Practically insoluble (NTP, 1992), Soluble in formic acid. Practically insoluble in ethanol, ether, chloroform, In water, 7.13X10+1 mg/L at 25 °C, 3.87e-02 g/L | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Off-white amorphous powder, Crystals from acetic acid and methanol | |
CAS No. |
31431-39-7 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=31431-39-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mebendazole [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0031431397 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757838 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=184849 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Mebendazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.046.017 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MEBENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/81G6I5V05I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
551.3 °F (NTP, 1992), 288.5 °C | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.