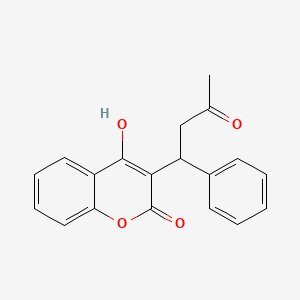
Warfarin
説明
Discovery and Early Applications as a Rodenticide
The discovery of this compound traces its origins to a devastating agricultural crisis that emerged in the early 1920s across the northern United States and Canada. Cattle herds began experiencing an unprecedented hemorrhagic disease characterized by fatal bleeding episodes following minor procedures or even spontaneously. The condition, which would later be termed "sweet clover disease," manifested most dramatically when cattle underwent routine procedures such as dehorning or castration, with mortality rates reaching alarming levels - in some documented cases, twenty-one of twenty-two cows died following dehorning procedures.
The breakthrough in understanding this mysterious cattle disease came through the dedicated work of veterinary pathologist Frank Schofield in 1921, who identified moldy silage made from sweet clover (Melilotus alba and Melilotus officinalis) as the causative agent. Schofield's systematic approach involved separating good clover stalks from damaged stalks within the same hay supply and feeding each to different experimental rabbits. The results were conclusive: rabbits consuming undamaged stalks remained healthy, while those ingesting damaged stalks developed fatal hemorrhagic illness. This experimental design provided the first definitive proof that spoiled sweet clover hay contained a potent anticoagulant factor.
The pivotal moment in this compound's discovery occurred during a severe blizzard in early 1933, when Wisconsin farmer Ed Carlson undertook a desperate two-hundred-mile journey from Deer Park to Madison, seeking answers for the mysterious deaths plaguing his cattle herd. Finding most veterinary offices closed on that Saturday, Carlson instead approached the laboratory of biochemist Karl Paul Link at the University of Wisconsin campus. Carlson's dramatic presentation to Link - consisting of a milk jug filled with unclotted blood, a deceased cow, and a pile of moldy hay - provided the essential materials that would launch the systematic chemical investigation leading to this compound's isolation.
Karl Link and his research team, including student assistant Eugen Wilhelm Schoeffel, immediately recognized the symptoms as characteristic of sweet clover disease and embarked on a comprehensive six-year research program to identify and isolate the active anticoagulant compound. Their methodical approach employed innovative in vitro clotting assays using rabbit plasma to guide the chemical fractionation of compounds extracted from the contaminated hay. By 1940, after extensive analytical work, Link's team successfully established that a naturally occurring substance called coumarin was being oxidized in moldy hay to produce 3,3′-methylene-bis(4-hydroxycoumarin), which would become known as dicoumarol.
The following table summarizes key developmental milestones in this compound's discovery and early rodenticide applications:
| Year | Milestone | Key Contributors | Significance |
|---|---|---|---|
| 1921 | Sweet clover disease identified | Frank Schofield | First identification of moldy clover as anticoagulant source |
| 1929 | Prothrombin deficiency demonstrated | Lee M. Roderick | Established mechanism of hemorrhagic disease |
| 1933 | Systematic research initiated | Karl Link, Ed Carlson | Beginning of comprehensive chemical investigation |
| 1940 | Dicoumarol isolated | Link research team | First pure anticoagulant compound extracted |
| 1948 | This compound commercialized | Wisconsin Alumni Research Foundation | Market introduction as rodenticide |
This compound's chemical structure, formally designated as 4-hydroxy-3-(3-oxo-1-phenylbutyl)-2H-1-benzopyran-2-one, belongs to the 4-hydroxycoumarin class of compounds. The molecule exists as a racemic mixture with the molecular formula Carbon nineteen Hydrogen sixteen Oxygen four and a molecular weight of 308.333 grams per mole. Its physical properties include formation of colorless crystals that are practically insoluble in water but readily soluble in organic solvents such as acetone and dioxane.
Transition to Therapeutic Use in Anticoagulation
The transformation of this compound from an exclusively pest control agent to a therapeutic anticoagulant emerged from an unexpected clinical incident that demonstrated both its potential dangers and therapeutic possibilities. In 1951, a young naval officer attempted suicide by consuming multiple doses of this compound rat poison over a five-day period. Rather than proving fatal, this incident provided valuable clinical data when the officer was successfully treated at a Naval hospital with vitamin K administration, making a complete recovery. This case demonstrated that this compound's anticoagulant effects could be effectively reversed, suggesting potential for controlled therapeutic applications.
The systematic evaluation of this compound for human therapeutic use began in earnest following this incident, with clinical researchers recognizing several advantages over existing anticoagulant medications. At the time, the primary anticoagulant options included heparin, which required parenteral administration, and dicoumarol, which exhibited a prolonged lag period before achieving therapeutic effect and presented stability challenges in clinical settings. This compound demonstrated superior characteristics including high oral bioavailability, excellent water solubility, and more predictable pharmacological effects compared to dicoumarol.
The development of this compound sodium, a water-soluble formulation suitable for both oral and intravenous administration, represented a crucial advancement in its therapeutic application. This formulation could be administered through multiple routes and monitored through routine blood testing procedures, offering significant practical advantages over existing anticoagulants that required intensive clinical supervision. The ability to reverse this compound's effects with vitamin K administration provided an additional safety margin that enhanced its clinical acceptability.
Clinical trials of this compound as a therapeutic anticoagulant commenced in 1941 at Wisconsin General Hospital and the Mayo Clinic, initially focusing on dicoumarol before expanding to include this compound sodium. These studies established this compound's efficacy in preventing thrombotic complications while demonstrating its superior pharmacological profile compared to existing alternatives. The research demonstrated that this compound could effectively prevent blood clot formation through inhibition of vitamin K-dependent clotting factors, providing therapeutic anticoagulation without the limitations associated with earlier compounds.
The mechanism underlying this compound's anticoagulant activity involves specific inhibition of vitamin K epoxide reductase complex subunit 1, an enzyme crucial for vitamin K recycling. Vitamin K serves as an essential cofactor in the gamma-carboxylation of coagulation factors VII, IX, X, and thrombin, a process that induces conformational changes allowing these factors to bind calcium and phospholipid surfaces. By irreversibly inhibiting vitamin K epoxide reductase, this compound prevents the conversion of vitamin K epoxide to vitamin K1, effectively disrupting the recycling pathway and creating a functional vitamin K deficiency.
The clinical approval of this compound sodium for human therapeutic use occurred in 1954, when it entered the market under the brand name Coumadin. This approval represented the culmination of over two decades of research and development, transforming a compound originally discovered through agricultural veterinary investigations into a cornerstone of anticoagulant therapy. The widespread acceptance of this compound in clinical practice was significantly enhanced in 1955 when President Dwight D. Eisenhower received this compound treatment following a highly publicized heart attack, effectively demonstrating its safety and efficacy to both medical professionals and the general public.
The following table presents the timeline of this compound's transition from rodenticide to therapeutic application:
| Year | Development | Clinical Significance | Impact |
|---|---|---|---|
| 1951 | Naval officer suicide attempt | Demonstrated reversibility with vitamin K | Established safety profile for human use |
| 1941-1954 | Clinical trials at Wisconsin General Hospital and Mayo Clinic | Proved therapeutic efficacy | Validated anticoagulant applications |
| 1954 | Food and Drug Administration approval | Authorized for human therapeutic use | Market introduction as Coumadin |
| 1955 | Presidential treatment | Enhanced public acceptance | Widespread clinical adoption |
The biochemical mechanism of this compound's anticoagulant action involves complex interactions with the vitamin K cycle, specifically targeting the enzyme vitamin K epoxide reductase. This mechanism creates a time-dependent anticoagulant effect, as existing clotting factors must be depleted before therapeutic anticoagulation is achieved. The half-lives of vitamin K-dependent clotting factors vary significantly: factor VII has a half-life of six hours, factors IX and X have half-lives of twenty-four and thirty-six hours respectively, while thrombin has a half-life of fifty hours. This temporal variation in factor depletion creates the characteristic delayed onset of this compound's therapeutic effect.
特性
IUPAC Name |
4-hydroxy-3-(3-oxo-1-phenylbutyl)chromen-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H16O4/c1-12(20)11-15(13-7-3-2-4-8-13)17-18(21)14-9-5-6-10-16(14)23-19(17)22/h2-10,15,21H,11H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PJVWKTKQMONHTI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)CC(C1=CC=CC=C1)C2=C(C3=CC=CC=C3OC2=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H16O4 | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5023742 | |
| Record name | Warfarin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023742 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
308.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Warfarin is an odorless and colorless solid. Used as a rodenticide for Norway rats and for house mice. (EPA, 1998), Colorless, odorless, crystalline powder. [rodenticide]; [NIOSH], Solid, COLOURLESS-TO-WHITE POWDER., Colorless, odorless, crystalline powder or solid., Colorless, odorless, crystalline powder. [Note: Rodenticide.] | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Warfarin | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/673 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | Warfarin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001935 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | WARFARIN | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/714 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
| Record name | Warfarin | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh/npg/npgd0665.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards is intended as a source of general industrial hygiene information on several hundred chemicals/classes for workers, employers, and occupational health professionals. Read more: https://www.cdc.gov/niosh/npg/ | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
Boiling Point |
Decomposes (NIOSH, 2023), decomposes, Decomposes | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | WARFARIN | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/714 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
| Record name | Warfarin | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh/npg/npgd0665.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards is intended as a source of general industrial hygiene information on several hundred chemicals/classes for workers, employers, and occupational health professionals. Read more: https://www.cdc.gov/niosh/npg/ | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
Solubility |
0.002 % (NIOSH, 2023), Solubility: 1 g in 1.5 mL water, 1.9 mL alcohol, >10,000 mL chloroform, >10,000 mL ether /Warfarin potassium/, In water, 17 mg/L at 20 °C, Soluble in acetone, dioxane; moderately soluble in methanol, ethanol, isopropanol, some oils; freely soluble in alkaline aqueous solution (forms a water-sol sodium salt); practically insoluble in benzene, cyclohexane, Skellysolves A and B., In acetone 65, chloroform 56, dioxane 100 (all in g/L, 20 °C), 0.017 mg/mL at 20 °C, Solubility in water, g/100ml at 20 °C: 0.0017 (very poor), 0.002% | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Warfarin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00682 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | WARFARIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Warfarin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001935 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | Warfarin | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh/npg/npgd0665.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards is intended as a source of general industrial hygiene information on several hundred chemicals/classes for workers, employers, and occupational health professionals. Read more: https://www.cdc.gov/niosh/npg/ | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
Density |
Relative density (water = 1): 1.4 | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
Vapor Density |
Relative vapor density (air = 1): 10.6 | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
Vapor Pressure |
0.09 mmHg at 71 °F (NIOSH, 2023), 0.09 [mmHg], 1.5X10-3 mPa /1.125X10-8 mm Hg/ at 25 °C, Vapor pressure, Pa at 20 °C: (negligible), 0.09 mmHg at 71 °F, (71 °F): 0.09 mmHg | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Warfarin | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/673 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
| Record name | WARFARIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | WARFARIN | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/714 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
| Record name | Warfarin | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh/npg/npgd0665.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards is intended as a source of general industrial hygiene information on several hundred chemicals/classes for workers, employers, and occupational health professionals. Read more: https://www.cdc.gov/niosh/npg/ | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
Color/Form |
Crystals from alcohol, White powder, The racemate forms colorless crystals | |
CAS No. |
81-81-2 | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | (±)-Warfarin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=81-81-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Warfarin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000081812 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Warfarin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00682 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | warfarin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757385 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | warfarin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=59813 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1-phenylbutyl)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Warfarin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023742 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Warfarin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.001.253 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | WARFARIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/5Q7ZVV76EI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | WARFARIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Warfarin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001935 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | WARFARIN | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/714 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
| Record name | Coumarin, 3-(alpha-acetonylbenzyl)-4-hydroxy- | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh-rtecs/GN456D70.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards (NPG) provides general industrial hygiene information for workers, employers, and occupational health professionals. It contains safety information and hazard data related to chemical substances or mixtures. | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
Melting Point |
322 °F (EPA, 1998), 161 °C, 322 °F | |
| Record name | WARFARIN | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/5240 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Warfarin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00682 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | WARFARIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1786 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Warfarin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001935 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | WARFARIN | |
| Source | ILO-WHO International Chemical Safety Cards (ICSCs) | |
| URL | https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0821 | |
| Description | The International Chemical Safety Cards (ICSCs) are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the Cards is to promote the safe use of chemicals in the workplace. | |
| Explanation | Creative Commons CC BY 4.0 | |
| Record name | WARFARIN | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/714 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
| Record name | Warfarin | |
| Source | The National Institute for Occupational Safety and Health (NIOSH) | |
| URL | https://www.cdc.gov/niosh/npg/npgd0665.html | |
| Description | The NIOSH Pocket Guide to Chemical Hazards is intended as a source of general industrial hygiene information on several hundred chemicals/classes for workers, employers, and occupational health professionals. Read more: https://www.cdc.gov/niosh/npg/ | |
| Explanation | The information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations. | |
準備方法
合成経路と反応条件
ワルファリンは、いくつかの方法で合成することができます。 一般的な合成経路の1つは、4-ヒドロキシクマリンとベンジリデンアセトンを塩基性条件下で縮合させることです 。反応には通常、水酸化ナトリウムを塩基として、エタノールを溶媒として使用します。生成物は再結晶によって精製されます。
工業的製造方法
ワルファリンの工業的製造には、実験室で使用される方法と同様の方法を使用した、大規模な合成が含まれます。 このプロセスは、収量と純度が最適化されており、多くの場合、連続フローリアクターと高度な精製技術が使用されています 。最終製品は、医療用タブレットまたは注射液に製剤化されます。
化学反応の分析
科学研究への応用
ワルファリンは、幅広い科学研究への応用があります。
科学的研究の応用
Warfarin has a wide range of scientific research applications:
作用機序
ワルファリンは、ビタミンKエポキシドレダクターゼ酵素を阻害することで抗凝固効果を発揮します 。この酵素は、ビタミンKエポキシドをその活性型であるビタミンKヒドロキノンに戻す役割を担っています。 この変換を阻害することにより、ワルファリンは活性型ビタミンKのレベルを低下させ、ビタミンK依存性凝固因子(II、VII、IX、およびX)の合成を減少させます 。これにより、血栓形成能力が低下します。
類似化合物との比較
Research Advances and Challenges
- AI-Driven Dosing : Machine learning algorithms predict this compound doses more accurately than physicians (84.0% vs. 81.9% within ±0.3 INR; P = 0.014), addressing its narrow therapeutic window .
- Genetic Testing: Pharmacogenomic-guided dosing improves this compound efficacy but is underutilized compared to DOACs’ simplicity .
生物活性
Warfarin, a widely used anticoagulant, plays a critical role in the prevention and treatment of thromboembolic disorders. Its primary mechanism involves the inhibition of vitamin K-dependent clotting factors. This article explores the biological activity of this compound, including its pharmacodynamics, pharmacokinetics, interactions, and effects beyond hemostasis.
This compound functions as a vitamin K antagonist by competitively inhibiting the vitamin K epoxide reductase complex subunit 1 (VKORC1). This inhibition prevents the conversion of vitamin K epoxide to its active form, thereby depleting vitamin K reserves necessary for synthesizing several clotting factors, including Factors II (prothrombin), VII, IX, and X.
Key Points:
- Vitamin K-Dependent Proteins : this compound affects the synthesis of not only coagulation factors but also proteins C and S, which are critical for anticoagulation.
- Prothrombotic State : The half-lives of vitamin K-dependent proteins lead to a temporary prothrombotic state during the initial days of therapy due to the faster degradation of proteins C and S compared to pro-coagulation factors .
Table 1: Half-Lives of Key Clotting Factors
| Clotting Factor | Half-Life (Hours) |
|---|---|
| Factor II (Prothrombin) | 50 |
| Factor VII | 6 |
| Factor IX | 24 |
| Factor X | 36 |
| Protein C | 8 |
| Protein S | 24 |
Pharmacokinetics
This compound is a racemic mixture consisting of two enantiomers: S-warfarin and R-warfarin. The S-enantiomer is significantly more potent (3-5 times) than the R-enantiomer and is primarily metabolized by cytochrome P450 enzymes, particularly CYP2C9 .
Absorption and Distribution:
- Bioavailability : Approximately 100% after oral administration.
- Distribution : Highly protein-bound (approximately 97%) primarily to albumin.
Metabolism:
- Major Pathway : Oxidation to various hydroxywarfarins by CYP2C9.
- Elimination Half-Life : Approximately 36 to 42 hours for the racemic mixture .
Biological Effects Beyond Hemostasis
Recent studies have highlighted this compound's effects on biological processes beyond coagulation. These include:
- Bone Health : this compound impacts bone growth and vascular calcification through its action on vitamin K-dependent proteins involved in these processes .
- Antitumor Activity : Some studies suggest that this compound may exhibit antitumor properties independent of its anticoagulant effects, possibly through modulation of non-haemostatic VKD proteins .
- Immunomodulatory Effects : this compound has been shown to influence inflammatory responses, indicating potential therapeutic applications in various pathological conditions .
Drug Interactions
This compound's efficacy can be significantly affected by various drug interactions. Co-administration with certain medications can enhance or inhibit its anticoagulant effect, leading to increased risks of bleeding or thrombosis.
Common Interactions:
- Increased Effect : Aspirin, metronidazole, and trimethoprim-sulfamethoxazole can potentiate this compound's effects.
- Decreased Effect : Drugs like phenobarbital and rifampicin may reduce this compound's anticoagulant activity .
Case Study Example:
A case study involving a patient on this compound therapy who developed significant bleeding complications after starting aspirin illustrates the importance of monitoring drug interactions closely. The patient's INR (International Normalized Ratio) was found to be significantly elevated due to the combined effects of both medications.
Q & A
Q. What computational frameworks improve this compound metabolite profiling in genotype-phenotype studies?
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


