Mebendazole
Description
Historical Context of Mebendazole Discovery and Development
This compound emerged from the research efforts at Janssen Pharmaceutica in Belgium, coming into use in 1971 wikipedia.org. It was developed as a synthetic broad-spectrum anthelmintic, active against a wide range of parasitic infestations researchgate.netmdpi.com. Its development marked a significant advancement in the treatment of helminthic diseases, offering a new therapeutic option for widespread parasitic infections. This compound was first approved for use in the United States in 1974 nih.gov.
Evolution of this compound's Therapeutic Applications
Initially, this compound's therapeutic applications were centered on treating common gastrointestinal parasitic infections caused by nematodes such as roundworms (Ascaris lumbricoides), hookworms (Necator americanus and Ancylostoma duodenale), pinworms (Enterobius vermicularis), and whipworms (Trichuris trichiura) wikipedia.orgresearchgate.netnih.gov. Its effectiveness against both larval and adult stages of nematodes, and in some cases, the eggs, established it as a valuable tool in antiparasitic chemotherapy wikipedia.org.
Over time, its use expanded to include other less common parasitic infections, such as capillariasis, cystic echinococcosis (Echinococcus granulosus), toxocariasis, trichinellosis (Trichinella spiralis), and trichostrongyliasis researchgate.netnih.gov. While primarily acting locally in the gut due to poor systemic absorption, research has explored its utility in cases where systemic activity might be beneficial, although other drugs are often preferred for infections outside the digestive tract wikipedia.orgnih.gov.
Current Scope of this compound Research in Diverse Fields
The scope of this compound research has expanded significantly beyond its traditional antiparasitic uses. A major area of current academic investigation is the potential repurposing of this compound as an anti-cancer agent researchgate.netclinicaltrials.euoup.comresearchgate.net. Preclinical studies have demonstrated its activity against various cancer cell lines and in animal tumor models, including brain tumors, lung cancer, ovarian cancer, and adrenocortical carcinoma researchgate.netoup.comecancer.orgnih.gov.
Research suggests that this compound's anti-cancer activity may be linked to its ability to inhibit microtubule polymerization in mammalian cells, similar to its effect on parasites, thereby disrupting cell division and potentially inducing apoptosis wikipedia.orgresearchgate.netnih.govmdpi.com. Studies are also exploring other potential mechanisms, such as the inhibition of angiogenesis and modulation of immune responses against tumors researchgate.netoup.com.
Furthermore, this compound is being investigated for potential therapeutic effects in other conditions, such as autosomal dominant polycystic kidney disease (ADPKD) and ulcerative colitis clinicaltrials.euguidetopharmacology.orgfrontiersin.org. Computational drug discovery approaches have identified this compound as a candidate for ADPKD treatment, with in vitro and in vivo studies suggesting its anti-cystic effect may involve microtubule polymerization inhibition and potential kinase target inhibition guidetopharmacology.orgfrontiersin.org. Research is also exploring its use in combination therapies for various conditions clinicaltrials.eu.
The following table summarizes some of the diverse research areas currently being explored for this compound:
| Research Area | Focus of Investigation |
| Oncology (Drug Repurposing) | Activity against various cancers (brain tumors, lung, ovarian, etc.); Mechanism of action (microtubule inhibition, anti-angiogenesis); Combination therapies. researchgate.netclinicaltrials.euoup.comresearchgate.netoup.comecancer.orgnih.gov |
| Autosomal Dominant Polycystic Kidney Disease (ADPKD) | Anti-cystic effects; Inhibition of microtubule polymerization; Potential kinase target inhibition. guidetopharmacology.orgfrontiersin.org |
| Ulcerative Colitis | Potential in managing symptoms, possibly in combination with other treatments. clinicaltrials.eu |
| Mechanism of Action | Detailed study of tubulin binding and microtubule disruption in parasites and mammalian cells. wikipedia.orgresearchgate.netnih.govmdpi.com |
| Combination Therapies | Exploring synergistic effects with other drugs for various conditions. researchgate.netclinicaltrials.eu |
Detailed research findings in oncology, for instance, include in vitro studies showing this compound's cytotoxic activity against glioblastoma cell lines at low micromolar concentrations and in vivo studies demonstrating extended survival in mouse models of glioma mdpi.comoup.comecancer.org. Research in ADPKD has shown that this compound can attenuate cystic growth in human cellular and mouse models guidetopharmacology.orgfrontiersin.org.
Academic research into this compound continues to reveal its potential beyond its established role as an anthelmintic, highlighting its versatility and the ongoing interest in exploring its full therapeutic potential.
Properties
IUPAC Name |
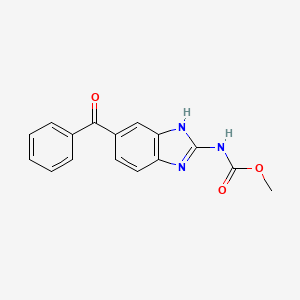
methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H13N3O3/c1-22-16(21)19-15-17-12-8-7-11(9-13(12)18-15)14(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,17,18,19,21) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OPXLLQIJSORQAM-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CC=C3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H13N3O3 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4040682 | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
295.29 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Mebendazole is a white to slightly yellow powder. Pleasant taste. Practically water insoluble. (NTP, 1992), Solid | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Practically insoluble (NTP, 1992), Soluble in formic acid. Practically insoluble in ethanol, ether, chloroform, In water, 7.13X10+1 mg/L at 25 °C, 3.87e-02 g/L | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Color/Form |
Off-white amorphous powder, Crystals from acetic acid and methanol | |
CAS No. |
31431-39-7 | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=31431-39-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Mebendazole [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0031431397 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757838 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | mebendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=184849 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Mebendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4040682 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Mebendazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.046.017 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MEBENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/81G6I5V05I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
551.3 °F (NTP, 1992), 288.5 °C | |
| Record name | MEBENDAZOLE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20586 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Mebendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00643 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | MEBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3232 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Mebendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014781 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Molecular and Cellular Mechanisms of Mebendazole Action
Targeting of β-Tubulin Polymerization
A key aspect of mebendazole's activity is its selective interaction with the β-tubulin subunit of microtubules in parasitic cells. This interaction disrupts the dynamic process of tubulin polymerization, which is vital for the formation and maintenance of the cellular cytoskeleton.
Binding to the Colchicine-Binding Site of β-Tubulin
This compound selectively binds to the colchicine-binding site on β-tubulin. wikipedia.orgdrugbank.comnih.govresearchgate.netcarehospitals.com This site is a specific region on the tubulin protein that is crucial for the assembly of tubulin dimers into microtubules. By occupying this site, this compound prevents the proper association of tubulin molecules. wikipedia.orgcarehospitals.compatsnap.com Research indicates that this compound effectively interacts with this site, with studies showing it can increase the thermal stability of β-tubulin, suggesting a direct binding interaction. kjpp.netresearchgate.net Molecular docking analyses further support that this compound fits within the colchicine-binding site of the α/β-tubulin heterodimer. kjpp.netresearchgate.net While this compound binds to the colchicine-binding site, its specific binding location within this site has been further characterized, with studies suggesting it binds to zones 2 and 3, located more deeply within the β-tubulin structure compared to colchicine which binds to zones 1 and 2. nih.gov This selective binding to parasite tubulin, with a significantly lower affinity for mammalian tubulin, contributes to the drug's selective toxicity against parasites. patsnap.compatsnap.com
Disruption of Cytoplasmic Microtubule Formation
The binding of this compound to the colchicine-binding site of β-tubulin directly inhibits the polymerization of tubulin dimers. wikipedia.orgpatsnap.com This blockage prevents the assembly of new microtubules and leads to the loss of existing cytoplasmic microtubules in the intestinal and tegumental cells of the parasites. wikipedia.orgdrugbank.comcarehospitals.comcambridge.orgpediatriconcall.com The disruption of microtubule formation is a principal mode of action for this compound. drugbank.compediatriconcall.com
Impact on Parasite Metabolism and Survival
Beyond its effects on microtubules, this compound significantly impacts the metabolic processes of parasites, particularly their ability to acquire and utilize glucose, which is their primary energy source. patsnap.com
Inhibition of Glucose Uptake
A direct consequence of microtubule disruption in the intestinal cells of parasites is the impaired uptake of glucose and other low molecular weight nutrients. wikipedia.orgdrugbank.comcarehospitals.compatsnap.compatsnap.comnih.govnih.govarpimed.ammedicinenet.com this compound causes selective and irreversible inhibition of glucose uptake in susceptible helminths. nih.govarpimed.am This inhibition is a key factor in starving the parasites of the energy required for their survival. patsnap.compatsnap.com Studies have shown that this compound causes a significant inhibitory effect on the glucose uptake of parasites in a time-dependent manner. cambridge.org While this compound inhibits glucose uptake, it does not inhibit glucose uptake in mammals. nih.gov
Impairment of Digestive and Reproductive Capacities
In helminths, this compound's primary mechanism involves binding to the colchicine-sensitive site of β-tubulin, inhibiting its polymerization into microtubules. drugbank.com This disruption of cytoplasmic microtubules leads to degenerative changes in the tegument and intestinal cells of the worm. drugbank.com A key consequence of microtubule loss is the impaired uptake of glucose by the larval and adult stages of susceptible parasites. drugbank.com This impaired glucose uptake depletes the helminth's glycogen stores, a vital energy reserve. drugbank.com The disruption of cellular structures and energy metabolism ultimately interferes with the parasite's digestive and reproductive functions, leading to immobilization, inhibition of egg production, and eventual death of the helminth. drugbank.comwho.intrxlist.commims.com
Disruption of Mitochondrial Function and ATP Production
This compound's effects extend to the mitochondria of helminths. Degenerative changes in the endoplasmic reticulum and the mitochondria of the germinal layer, coupled with the subsequent release of lysosomes, contribute to a decreased production of adenosine triphosphate (ATP). drugbank.com ATP is essential for the survival and energy requirements of the helminth. drugbank.com Diminished energy production due to mitochondrial disruption further contributes to the parasite's immobilization and eventual demise. drugbank.com Studies have shown that this compound can repress mitochondrial activity and reduce ATP concentration in cells, with observed downregulation of electron transport chain enzymes like ATP synthase and Cyt C in some organisms. nih.gov This suggests a direct impact on the energy production machinery within the parasite.
Mechanisms Beyond Tubulin Inhibition in Cancer Research
While the inhibition of tubulin polymerization is a well-established mechanism of this compound, research in cancer has revealed additional mechanisms that contribute to its anti-cancer properties. indexarepository.comnih.gov These mechanisms involve the modulation of various signaling pathways critical for cancer cell survival, proliferation, and metastasis. indexarepository.commdpi.comrjptonline.org
Modulation of Signaling Pathways
This compound has been shown to modulate several signaling pathways in cancer cells, influencing their behavior and response to therapy. rjptonline.orgaging-us.comnih.gov
Studies have indicated that this compound can activate the MEK-ERK pathway in certain cell types, such as monocytes and macrophages. aging-us.comresearchgate.net This activation has been suggested to have immunomodulating effects. researchgate.net In the context of cancer, while some studies suggest this compound can inhibit the MAPK/ERK pathway in specific cancer cell lines, particularly in combination with other inhibitors like trametinib, its effect on this pathway can be context-dependent. nih.govoncotarget.com For instance, in melanoma cells with NRAS mutations, this compound in combination with trametinib significantly decreased MEK and ERK phosphorylation. oncotarget.com
This compound has been shown to modulate several cancer-associated pathways, including AP1, STAT1/2, ELK1/SRF, and MYC/MAX. rjptonline.orgaging-us.comnih.govcancerchoices.orgnih.gov The regulatory outcomes on these pathways can be context-dependent, varying between different cancer cell lines. nih.govnih.gov For example, studies in head and neck squamous cell carcinoma (HNSCC) cell lines demonstrated that this compound could modulate these pathways. nih.govnih.gov In cisplatin-resistant ovarian cancer cells, this compound was shown to inhibit multiple cancer-related signal pathways, including ELK/SRF, MYC/MAX, and AP1. aging-us.com
Here is a summary of the reported modulation of these pathways by this compound:
| Pathway | Reported Effect in Cancer Cells | Reference(s) |
| AP1 | Modulated/Inhibited | rjptonline.orgaging-us.comnih.govcancerchoices.orgnih.gov |
| STAT1/2 | Modulated | rjptonline.orgaging-us.comnih.govcancerchoices.orgnih.gov |
| ELK1/SRF | Modulated/Inhibited | rjptonline.orgaging-us.comnih.govcancerchoices.orgnih.gov |
| MYC/MAX | Modulated/Inhibited | rjptonline.orgaging-us.comnih.govcancerchoices.orgnih.gov |
Recent research has identified a novel mechanism of action for this compound in ovarian cancer cells involving the Girdin-mediated Akt/IKKα/β/NF-κB signaling axis. mdpi.comnih.govdntb.gov.uaresearchgate.net Studies have shown that this compound treatment leads to a significant downregulation of Girdin, a known Akt modulator. mdpi.comnih.govresearchgate.net This downregulation subsequently results in reduced phosphorylation of Akt, IKKα/β, and NF-κB. mdpi.comnih.govresearchgate.net The inhibition of this signaling axis contributes to this compound's anti-cancer effects, including the suppression of cell proliferation, migration, and cancer stemness, as well as the induction of apoptosis in ovarian cancer cells. mdpi.comnih.govresearchgate.net This highlights the potential of targeting Girdin and its downstream signaling cascade as a therapeutic strategy in ovarian cancer. mdpi.comnih.gov
SK1 Inhibition and Sphingolipid Modulation
This compound has been shown to impede the proliferation and migration of pancreatic cancer cells through a pathway dependent on the inhibition of Sphingosine Kinase 1 (SK1). MBZ selectively inhibits SK1 more than SK2 and regulates the levels of sphingolipids. mdpi.comnih.govresearchgate.net Sphingosine 1-phosphate (S1P), a product of SK activity, plays a vital role in cancer growth, metastasis, chemotherapy, and drug resistance. mdpi.comresearchgate.net MBZ inhibits S1P-induced cancer cell growth and migration. mdpi.comnih.govresearchgate.net This inhibitory effect involves the regulation of the JAK2/STAT3/Bcl-2 pathway and the S1P/FAK/vimentin pathway. mdpi.comresearchgate.net Inhibition of S1P production by MBZ leads to the inhibition of FAK phosphorylation, which in turn suppresses the expression of vimentin, a protein involved in cancer cell migration. mdpi.com
Influence on Cancer-Associated Proteins and Genes
This compound influences the expression and activity of several proteins and genes implicated in cancer progression.
Inhibition of Hsp90
Recent experimental evidence suggests that this compound binds to Heat Shock Protein 90 (Hsp90) and inhibits acute myeloid leukemia cell growth. chemrxiv.orgresearchgate.net Hsp90 is a chaperone protein essential for the stability and function of numerous client proteins, many of which are critically involved in oncogenesis. chemrxiv.orgmdpi.com Computational studies, including molecular dynamics simulations, support the hypothesis that this compound is able to bind to the ATP binding site of Hsp90. chemrxiv.orgresearchgate.net This inhibition can lead to the proteasomal degradation of Hsp90 client proteins, such as GLI transcription factors, which are fundamental for the pathophysiology of leukemia initiating cells. chemrxiv.orgmdpi.com
Modulation of Glycolytic Targets (SLC2A1, HK1, GAPDH, LDHA)
Here is a table summarizing the glycolytic targets modulated by this compound:
| Target Gene | Protein Name | Role in Glycolysis | Effect of this compound |
| SLC2A1 | Glucose Transporter 1 (GLUT1) | Facilitates glucose uptake into cells. | Modulation of mRNA expression nih.govresearchgate.netresearchgate.netufc.br |
| HK1 | Hexokinase 1 | Catalyzes the first step of glycolysis. | Modulation of mRNA expression nih.govresearchgate.netresearchgate.netufc.br |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | Catalyzes an intermediate step in glycolysis. | Modulation of mRNA expression nih.govresearchgate.netresearchgate.netufc.br |
| LDHA | Lactate Dehydrogenase A | Converts pyruvate to lactate, regenerating NAD+. | Modulation of mRNA expression nih.govresearchgate.netresearchgate.netufc.br, Alters enzymatic activity nih.govresearchgate.net |
Effect on Matrix Metalloproteinases (MMPs)
This compound has been shown to suppress the activity of Matrix Metalloproteinase-9 (MMP-9) in ovarian cancer cells. mdpi.comnih.govdntb.gov.uasciforum.net MMPs are enzymes that contribute to ovarian cancer metastasis by degrading the extracellular matrix. sciforum.net By inhibiting MMP-9 activity, MBZ impedes the invasive potential of cancer cells. mdpi.comnih.govdntb.gov.uasciforum.net Studies in ovarian cancer cell lines have demonstrated that MBZ significantly impedes migration and spheroid invasion, which is accompanied by the suppression of MMP-9 activity. mdpi.comnih.govdntb.gov.uasciforum.net
Induction of Apoptosis and Cell Cycle Arrest
This compound induces apoptosis and cell cycle arrest in various cancer cell lines. mdpi.comnih.govdntb.gov.uaresearchgate.netrjptonline.orgbioscientifica.comresearchgate.net The induction of apoptosis by MBZ is a significant contributor to its anticancer effects. rjptonline.orgresearchgate.net This process can occur through both p53-dependent and independent pathways, and involves the activation of caspases, such as caspase-3, caspase-8, and caspase-9. ecancer.orgbioscientifica.com MBZ can disrupt mitochondrial membrane potential, triggering apoptosis. mdpi.comdntb.gov.uasciforum.net In melanoma cells, MBZ induces apoptosis through a BCL2-dependent mechanism and promotes the interaction of SMAC/DIABLO with XIAP, further facilitating apoptosis. nih.govaacrjournals.orgecancer.org
In addition to inducing apoptosis, this compound causes cell cycle arrest, primarily in the G2/M phase. mdpi.comnih.govdntb.gov.uaresearchgate.netrjptonline.orgbioscientifica.comresearchgate.net This arrest is associated with the modulation of key cell cycle regulatory proteins, including Cyclin B1, CDC25C, and WEE1. mdpi.comnih.govdntb.gov.ua By halting the cell cycle, MBZ prevents cancer cells from undergoing proliferation. rjptonline.orgresearchgate.net Studies in thyroid cancer cell lines, for example, have shown that MBZ significantly increases the percentage of cells in the G2/M phase in a dose-dependent manner. bioscientifica.com
Here is a table summarizing the effects of this compound on cell cycle phases in thyroid cancer cell lines:
| Cell Line | This compound Concentration | Percentage of Cells in G2/M Phase (vs. Control) | Citation |
| B-CPAP | 0.5 µM | Increased by 8.9% (P=0.0036) | bioscientifica.com |
| B-CPAP | 1 µM | Increased by 44.9% (P<0.0001) | bioscientifica.com |
| 8505c | 0.5 µM | Increased by 1% (ns) | bioscientifica.com |
| 8505c | 1 µM | Increased by 9.9% (P=0.0204) | bioscientifica.com |
*ns: not significant
Mitotic Arrest
Disruption of microtubule dynamics by this compound leads to the inability of cells to form a functional mitotic spindle. This results in the arrest of cells during the mitotic phase of the cell cycle wikipedia.orgecancer.orgresearchgate.net. Studies have shown that exposure of various cancer cell lines to this compound induces tubulin depolymerization, subsequently causing mitotic arrest. For instance, research on human non-small cell lung cancer (NSCLC) cell lines demonstrated that this compound induced tubulin depolymerization, leading to mitotic arrest ecancer.orgresearchgate.net. This mitotic arrest is a critical step preceding the induction of apoptosis in susceptible cells.
Caspase-Dependent Apoptosis
Following mitotic arrest, this compound has been shown to induce programmed cell death, or apoptosis, through pathways that involve caspases. Caspases are a family of proteases that play a central role in the execution phase of apoptosis. This compound-induced apoptosis is reported to be mediated via the activation of caspases, including caspase-3 researchgate.netbioscientifica.comspringermedizin.de. The mechanism can involve the dephosphorylation of Bcl-2, an anti-apoptotic protein, which then allows pro-apoptotic proteins like Bax to dimerize and initiate the apoptotic cascade wikipedia.org. Studies in thyroid cancer cell lines, such as papillary (B-CPAP) and anaplastic (8505c) cells, showed that this compound induced late-stage apoptosis through the activation of the caspase-3 pathway bioscientifica.com. Similarly, research on ovarian cancer cell lines indicated that this compound triggered apoptosis nih.gov.
G2/M Cell Cycle Arrest
In addition to mitotic arrest, this compound has been observed to induce cell cycle arrest at the G2/M phase. This arrest point is crucial as it precedes entry into mitosis. By interfering with microtubule formation, this compound prevents cells from progressing from the G2 phase to the M phase of the cell cycle researchgate.netbioscientifica.comspringermedizin.denih.govresearchgate.netthieme-connect.com. Studies in human papillary and anaplastic thyroid cancer cell lines demonstrated that this compound increased the percentage of cells in the G2/M phase bioscientifica.com. In ovarian cancer cell lines, this compound induced G2/M cell cycle arrest by modulating key cell cycle regulators such as Cyclin B1, CDC25C, and WEE1 nih.govresearchgate.net. This G2/M arrest is a direct consequence of the disrupted microtubule network necessary for proper chromosome segregation during mitosis.
Table 1: this compound-Induced G2/M Cell Cycle Arrest in Thyroid Cancer Cell Lines
| Cell Line | This compound Concentration | Percentage of Cells in G2/M Phase (vs. Vehicle Control) | Statistical Significance |
| B-CPAP | 0.5 µM | Increased by 8.9% | P = 0.0036 bioscientifica.com |
| 8505c | 0.5 µM | Increased by 1% | ns bioscientifica.com |
| B-CPAP | 1 µM | Increased by 44.9% | P < 0.0001 bioscientifica.com |
| 8505c | 1 µM | Increased by 9.9% | P = 0.0204 bioscientifica.com |
Data derived from research on human papillary (B-CPAP) and anaplastic (8505c) thyroid cancer cell lines treated with this compound for 24 hours bioscientifica.com.
Anti-angiogenic Effects
Angiogenesis, the formation of new blood vessels, is a critical process in the growth and progression of certain diseases. This compound has demonstrated anti-angiogenic properties in various studies ecancer.orgspringermedizin.demdpi.com. While the precise mechanisms can vary depending on the context, one proposed mechanism involves the reduction of vascular endothelial growth factor (VEGF) levels ecancer.org. VEGF is a key signaling protein that promotes the formation of blood vessels. By potentially downregulating VEGF, this compound can inhibit the development of new vasculature, thereby limiting nutrient and oxygen supply to rapidly growing tissues ecancer.orgwaocp.orgbiointerfaceresearch.com. Studies have shown that this compound reduced levels of VEGF in co-culture systems involving peripheral blood mononuclear cells (PBMCs), cancer cells, and endothelial cells nih.gov.
Immunomodulatory Activities of this compound
Beyond its direct effects on cellular processes and angiogenesis, this compound has also been reported to possess immunomodulatory activities mdpi.comnih.govresearchgate.netmdpi.comoncotarget.com. These effects can influence the immune response, particularly the function of monocytes and macrophages.
Induction of Pro-inflammatory M1 Phenotype in Monocytes/Macrophages
Macrophages exhibit significant plasticity and can differentiate into different phenotypes, broadly classified as pro-inflammatory (M1) and anti-inflammatory (M2). M1 macrophages are typically associated with anti-tumor immunity and the production of pro-inflammatory cytokines. Research indicates that this compound can induce the polarization of monocytes and macrophages towards a pro-inflammatory M1 phenotype mdpi.comnih.govresearchgate.netmdpi.comoncotarget.com. This shift in polarization can enhance the ability of these immune cells to exert anti-tumor effects nih.govresearchgate.netoncotarget.com. Studies using THP-1 monocytes and macrophages, as well as co-cultures with cancer cells, have shown that this compound can induce the release of pro-inflammatory cytokines such as TNF-alpha and IL-1beta, consistent with an M1 phenotype nih.govresearchgate.net. This induction of an M1 phenotype is suggested to contribute to the observed anti-tumor activity of this compound in some models mdpi.comresearchgate.net.
Table 2: this compound-Induced Pro-inflammatory Cytokine Release
| Cell Type | This compound Treatment | Cytokines Measured | Observed Effect |
| THP-1 monocytes/macrophages | Varied concentrations | IL-1β, TNF-alpha | Increased release of pro-inflammatory cytokines. nih.govresearchgate.net |
| PBMCs + Cancer/Endothelial cells | 0.3-10 µM | TNFα, IFNγ | Significant increases, indicating immune stimulation. researchgate.net |
Data compiled from studies investigating the immunomodulatory effects of this compound on monocytes and macrophages.
Modulation of Cytokine Levels (e.g., TNFα, IFNγ, IL-1β, IL-6)
This compound has been shown to modulate the release of several pro-inflammatory cytokines. In studies using human peripheral blood mononuclear cells (PBMCs) activated by IL2 and anti-CD3 stimulation, MBZ at concentrations of 1-10 µM stimulated the release of TNFα, IL1β, IFNγ, and IL6. nih.gov This effect was less pronounced in non-activated PBMCs. nih.gov Similarly, in co-culture systems involving T-cell receptor activated PBMCs and cancer cells, MBZ at concentrations ranging from 0.3 to 10 µM induced significant increases in TNFα and IFNγ, indicative of immune stimulation. nih.gov The release of IL1β, TNFα, and IL6 was attenuated in CD14-depleted PBMC cultures compared to intact cultures, suggesting a role for CD14 positive cells (monocytes/macrophages) in this process. nih.gov
The modulation of cytokine levels by MBZ appears to be concentration-dependent and can be influenced by co-stimulatory signals. At high concentrations, MBZ substantially induced the release of IL-1β, which was further potentiated by lipopolysaccharide (LPS). figshare.com At lower MBZ concentrations, co-treatment with LPS was required for stimulated IL-1β secretion. figshare.com
| Cytokine | Effect of this compound | Context | Source |
| TNFα | Increased release | Activated PBMCs, PBMC/tumor cell co-cultures | nih.gov |
| IFNγ | Increased release | Activated PBMCs, PBMC/tumor cell co-cultures | nih.gov |
| IL-1β | Increased release | Activated PBMCs, THP-1 cells | nih.govfigshare.com |
| IL-6 | Increased release | Activated PBMCs, THP-1 cells | nih.gov |
Activation of Inflammasomes (e.g., NLRP3)
Research indicates that this compound can induce the activation of inflammasomes, particularly the NLRP3 inflammasome. MBZ-induced IL-1β release has been found to be dependent on NLRP3 inflammasome activation. nih.govfigshare.comtandfonline.com This activation process has been shown to involve the stimulation of Toll-Like Receptor 8 (TLR8) and require the activation of protein kinase C, ERK1/2, and NF-kappaB. figshare.comtandfonline.com The activation of the NLRP3 inflammasome by MBZ contributes to the release of pro-inflammatory cytokines like IL-1β. figshare.commdpi.comfrontiersin.org
Toll-Like Receptor 8 (TLR8) Stimulation
Toll-Like Receptor 8 (TLR8) stimulation has been identified as a factor involved in this compound's mechanism of action, particularly in the context of inflammasome activation and cytokine release. MBZ-induced IL-1β release is reported to involve TLR8 stimulation. nih.govfigshare.comtandfonline.com Studies suggest that at higher concentrations (> 3 µM), MBZ can activate TLR8, contributing to the signals required for inflammasome activation and subsequent cytokine production. nih.gov
Enhancement of T-cell Activation and Tumor Cell Killing
This compound has demonstrated the ability to enhance T-cell activation and promote tumor cell killing, particularly in co-culture systems involving immune cells and cancer cells. MBZ potentiated the anticancer activity of CD3/IL2 activated PBMCs. nih.govnih.gov This effect was observed as a significant increase in tumor cell apoptosis and a reduction in the number of surviving tumor cells when PBMCs were co-cultured with lung cancer cells. nih.govnih.gov The enhancement of tumor cell killing by MBZ in this context was dependent on the presence of CD14 positive cells (monocytes/macrophages) in the co-culture, suggesting a role for these myeloid cells in mediating the T-cell-dependent anti-tumor effect. nih.govnih.gov MBZ stimulates CD14+ myeloid cells, which in turn enhance T-cell activation and tumor cell killing. nih.govnih.gov
Impact on Innate Immunity
This compound has been shown to impact innate immunity. Studies suggest that MBZ can induce a pro-inflammatory tumor-suppressive M1 phenotype in monocytes and macrophages. nih.govfigshare.comresearchgate.net This polarization towards an M1 phenotype is associated with phagocytic and antigen-presenting activity, production of Th-1 activating cytokines, and induction of cytotoxic effects. nih.gov M1 macrophages can also activate other immune cells, such as natural killer (NK) and T lymphocytes. nih.gov this compound therapy has been associated with increased innate immunity in clinical settings. researchgate.netnih.govmdpi.com
Clinical Efficacy and Drug Repurposing Research
Efficacy in Parasitic Infections
Mebendazole demonstrates efficacy against a range of parasitic worm infections, primarily those affecting the gastrointestinal tract. nih.govyashodahospitals.comwikipedia.org Its effectiveness can vary depending on the targeted helminth species and the specific parasitic condition. parahostdis.orgplos.org
Broad-Spectrum Anthelmintic Activity
This compound is classified as a broad-spectrum synthetic anthelmintic. drugbank.comyashodahospitals.com It is effective against single or mixed helminthic infestations. hres.ca Its activity extends to both adult and immature larval stages of nematodes, and it can also kill the eggs of some species, such as roundworm and whipworm. wikipedia.org
Targeted Helminth Species (e.g., Ascaris lumbricoides, Enterobius vermicularis, Necator americanus, Ancylostoma duodenale, Trichuris trichiura)
This compound is indicated for the treatment of infections caused by several specific helminth species. These include Enterobius vermicularis (pinworm), Ascaris lumbricoides (common roundworm), Trichuris trichiura (whipworm), Ancylostoma duodenale (common hookworm), and Necator americanus (American hookworm) in both single and mixed infections. nih.govdrugbank.comhres.cadroracle.ai
Clinical studies have evaluated the efficacy of this compound against these targeted species, with varying cure rates and egg reduction rates reported depending on factors such as the study population, infection intensity, and treatment regimen. parahostdis.orgplos.orgdroracle.ainih.govoamjms.eu
| Helminth Species | Reported Cure Rate (CR) | Reported Egg Reduction Rate (ERR) | Source Indices |
| Enterobius vermicularis | Mean 95% | Not specified | droracle.ai |
| Ascaris lumbricoides | 89.7% to 100% | 98.6% to 99.9% | parahostdis.org |
| Trichuris trichiura | 44.4% to 90.0% | 96.4% to 99.6% | parahostdis.orgnih.gov |
| Ancylostoma duodenale | 71.4% | 97.3% | parahostdis.org |
| Necator americanus | 52.0% to 22.9% | 91.2% | parahostdis.orgnih.gov |
Note: Efficacy can vary based on study design, dosage, and geographical region.
Research indicates that while this compound is highly effective against Ascaris lumbricoides and Enterobius vermicularis, its efficacy against hookworms, particularly Necator americanus, and Trichuris trichiura can be lower. parahostdis.orgplos.orgoamjms.eu For instance, a study involving schoolchildren in six countries reported an estimated Fecal Egg Count Reduction (FECR) rate for this compound of 97.6% for A. lumbricoides, 79.6% for hookworm, and 63.1% for T. trichiura. plos.org Another study comparing albendazole and this compound found that while albendazole had better efficacy against A. lumbricoides, this compound was more effective against T. trichiura in terms of cure rate in one study, although other studies showed similar or lower efficacy for this compound against T. trichiura. oamjms.eudroracle.ai
Efficacy Against Specific Parasitic Conditions (e.g., Capillariasis, Echinococcosis, Toxocariasis, Trichinellosis, Trichostrongyliasis, Giardia lamblia)
Beyond the common soil-transmitted helminths, this compound has been used off-label for the treatment of other parasitic conditions. nih.gov These include intestinal capillariasis (Capillaria philippinensis), echinococcosis (cystic, caused by Echinococcus granulosus), toxocariasis, trichinellosis (Trichinella spiralis), and trichostrongyliasis. nih.govnafdac.gov.ng
Studies have investigated the effectiveness of this compound in these less common infections. For echinococcosis, this compound has shown some effectiveness, particularly against the germinal tissue of E. granulosus cysts, although complete eradication can be challenging, and effectiveness may depend on drug formulation and administration route. bibliotekanauki.plnih.gov For capillariasis, this compound has been suggested as a treatment option. nafdac.gov.ngdrugfuture.com this compound has also been used for toxocariasis and trichinellosis. nih.govnafdac.gov.ng While this compound has been used for giardiasis (Giardia lamblia), it is not considered the preferred agent for this infection. wikipedia.orgmedscape.com
Resistance Mechanisms and Surveillance
The widespread use of benzimidazole anthelmintics like this compound has raised concerns about the development of drug resistance in helminth populations. parahostdis.orgnih.govnih.govfrontiersin.org While definitive evidence of clinically relevant benzimidazole resistance in humans is not clearly documented globally, reduced efficacy has been observed in some regions and helminth species. nih.govfrontiersin.org
Changes in β-Tubulin Protein
A primary mechanism of resistance to benzimidazole drugs, including this compound, involves changes in the parasite's β-tubulin protein. nih.govdroracle.aikjpp.netnih.govcambridge.orgresearchgate.net Benzimidazoles exert their effect by binding to the colchicine-sensitive site of β-tubulin, inhibiting its polymerization and disrupting microtubule formation. nih.govdrugbank.comkjpp.netnih.govresearchgate.net Resistance can arise from single nucleotide polymorphisms (SNPs) in the β-tubulin isotype 1 gene, which alter the binding site and reduce the drug's affinity for tubulin. cambridge.orgmdpi.com Specific mutations at codons 167, 198, and 200 of the β-tubulin gene have been associated with benzimidazole resistance in various helminth species, including those infecting humans and livestock. cambridge.orgmdpi.com These genetic changes can lead to reduced drug efficacy. cambridge.org
Emergence of Drug Resistance in Specific Regions or Helminth Species
Reports suggest the potential emergence of drug resistance in certain human helminth species and geographical areas, particularly where mass drug administration programs have been implemented over extended periods. parahostdis.orgnih.govnih.govfrontiersin.org Reduced efficacy of this compound against hookworms, particularly Necator americanus, has been reported in some regions, such as Mali and Pemba Island. parahostdis.orgnih.gov There are also concerns about decreasing efficacy against Trichuris trichiura. parahostdis.orgnih.govfrontiersin.org
Studies have shown a decline in the cure rates and egg reduction rates of this compound against hookworms and T. trichiura over time in certain areas, which could indicate the development of resistance. nih.govfrontiersin.org While the presence of resistance-encoding SNPs in human helminths has been investigated in some regions, the direct link between these mutations and observed clinical resistance in humans is still an area of ongoing research. frontiersin.orgmdpi.com The patterns of resistance development seen in veterinary helminths, where benzimidazole resistance is a significant issue and associated with specific β-tubulin mutations, raise concerns for human helminth control programs. nih.govfrontiersin.orgcambridge.org
This compound Repurposing in Oncology
This compound (MBZ), an anthelmintic drug with a long history of use for parasitic infections, has garnered significant attention for its potential as a repurposed anticancer agent. Preclinical studies have explored its efficacy across a range of cancer types, demonstrating various mechanisms of action, including tubulin disruption, inhibition of angiogenesis, induction of apoptosis, and modulation of signaling pathways. The repurposing of existing drugs like this compound offers a potentially faster and more cost-effective path to identifying new cancer therapies.
Preclinical Studies in Various Cancer Types
Preclinical research involving both in vitro (cell line) and in vivo (animal model) studies has investigated the anticancer properties of this compound. These studies aim to understand the drug's effects on cancer cell growth, survival, migration, and tumor progression.
This compound has shown promising preclinical efficacy in models of brain tumors, including gliomas and meningiomas. Studies have indicated that this compound can penetrate the blood-brain barrier, which is crucial for treating central nervous system malignancies.
Gliomas: In glioblastoma multiforme (GBM) cell lines, this compound has demonstrated cytotoxicity with half-maximal inhibitory concentrations (IC50) typically ranging from 0.1 to 0.3 µM. This compound disrupted microtubule formation in GBM cells, and this in vitro activity correlated with reduced tubulin polymerization. In vivo studies using syngeneic and xenograft orthotopic mouse glioma models showed that this compound significantly extended mean survival, in some cases up to 63%. This compound has also been shown to inhibit glioblastoma cell proliferation, an effect that was enhanced when combined with chloroquine and further augmented with temozolomide. Different polymorphs of this compound exist, and studies suggest that polymorph C is more efficacious in brain tumor therapy, reaching therapeutically effective concentrations in brain tissue and tumors in mouse models. Combining this compound polymorph C with elacridar, a P-glycoprotein inhibitor, further improved efficacy in mouse glioma and medulloblastoma models. This compound also sensitized interphase glioma cells to the effects of ionizing radiation.
Meningioma: Preclinical studies in malignant meningioma models have shown that this compound, both alone and in combination with radiation, can increase survival. In vitro experiments on meningioma cell lines revealed IC50 values for this compound in the range of 0.26-0.42 µM. The combination of this compound and radiation resulted in a greater reduction in colony formation and higher levels of cleaved caspase-3, a marker of apoptosis. In vivo studies demonstrated that this compound alone and the combination led to increased apoptosis and decreases in tumor cell and vascular proliferation. This combination increased median survival and delayed tumor growth by inducing apoptosis via the caspase-3/7 pathway, decreasing cell proliferation, and reducing levels of the angiogenesis marker CD31.
Here is a summary of some preclinical findings in brain tumors:
| Cancer Type | Model (In vitro/In vivo) | Key Findings | Citations |
| Glioblastoma | Cell lines (In vitro) | Cytotoxicity (IC50 0.1-0.3 µM), disrupted microtubule formation. | |
| Glioblastoma | Mouse models (In vivo) | Extended mean survival (up to 63%), inhibited tumor growth. | |
| Glioblastoma | Cell lines (In vitro) | Inhibition of proliferation, enhanced by chloroquine and temozolomide. | |
| Glioblastoma |
Colorectal Cancer
Research suggests this compound exhibits preclinical anti-cancer activity in colorectal cancer. oncotarget.comnih.gov Studies have indicated its potential to inhibit cancer cell proliferation and induce apoptosis in colorectal cancer cell lines. droracle.ai In vitro and in vivo studies have demonstrated anticancer activity against colorectal cancer cells, with reported IC50 values providing an indication of its potency. droracle.ai
In a preclinical model of colon cancer chemoprevention using ApcMin/+ mice, this compound was shown to slow colon cancer xenograft growth when used therapeutically. oncotarget.comnih.gov The combination of this compound and sulindac significantly reduced microadenomas, polyp number, and size in the intestines of these mice. oncotarget.comnih.gov this compound as a single agent decreased COX2 expression, blood vessel formation, and VEGFR2 phosphorylation in this model. oncotarget.com
For metastatic colorectal cancer, adding this compound to standard chemotherapy regimens has shown potential in improving response rates and extended progression-free survival in some clinical observations. droracle.ai417integrativemedicine.com One case study reported a man with metastatic colon cancer achieving partial remission after treatment with this compound following progression on conventional therapies. cancerchoices.org
Lung Cancer (e.g., Non-Small Cell Lung Cancer)
This compound has demonstrated cytotoxic effects on non-small cell lung cancer (NSCLC) cell lines, including A549, H1299, and H460. nih.govmdpi.comrjptonline.org Studies have shown that this compound can hinder the growth of lung cancer cells in laboratory experiments and in living organisms. rjptonline.org It has been observed to induce dose-dependent apoptotic responses in lung cancer cell lines. ecancer.orgnih.gov
In vivo studies using mouse xenografts of H460 NSCLC cells showed that oral treatment with this compound resulted in a dose-dependent arrest in tumor growth. nih.govnih.gov Mice treated with this compound showed a significantly lower mean colony count of lung metastases compared to controls. mdpi.comecancer.orgnih.gov The proposed molecular mechanism includes the stabilization of post-translational p53 and the downstream expression of p21 and MDM2 in lung cancer cell lines. mdpi.comecancer.org this compound can induce mitotic arrest and apoptosis by depolymerizing tubulin in NSCLC cells. mdpi.com Recent research indicates that this compound inhibited NSCLC cell proliferation and migration while promoting apoptosis through triggering ROS generation and abrogating the JAK2-STAT3 signaling pathway. researchgate.net
Melanoma
This compound has shown activity against melanoma cell lines, including chemoresistant lines. parahostdis.orgecancer.orgmdpi.comeurekalert.orgaacrjournals.org Screening studies identified this compound as having significant growth-inhibitory effects on melanoma cells with lower IC50 values compared to non-cancerous melanocytes. ecancer.orgmdpi.comeurekalert.orgaacrjournals.org
| Cell Line | This compound IC50 (µM) | Source |
| SK-Mel-19 | 0.32 | mdpi.comaacrjournals.org |
| M-14 | 0.30–0.32 | mdpi.comaacrjournals.org |
| Melan-a | 1.9 | aacrjournals.org |
In vitro studies have shown that this compound treatment of melanoma cells resulted in a time-dependent decrease in XIAP levels, correlating with increased apoptosis markers. mdpi.com this compound induced apoptosis through phosphorylation of Bcl-2 and activation of both intrinsic and extrinsic mitochondrial pathways in melanoma cells. mdpi.comeurekalert.orgaacrjournals.org
In vivo studies using human melanoma xenografts in mice demonstrated that this compound inhibited tumor growth effectively, comparable to temozolomide, the standard treatment for melanoma, without observed toxicity. ecancer.orgmdpi.comresearchgate.netresearchgate.netascopubs.org Oral administration of this compound resulted in Bcl-2 phosphorylation in vivo, supporting its mechanism of action. researchgate.netascopubs.org
Pancreatic Cancer
Preclinical studies have explored this compound's potential in pancreatic cancer. canceractive.comrjptonline.orghopkinsmedicine.orgnih.govmdpi.com Research using mouse models of pancreatic cancer has indicated that this compound could slow or stop the growth and spread of both early and late-stage pancreatic cancer, particularly being effective for early-detected cancer. canceractive.comhopkinsmedicine.org
Studies suggest that this compound may act by collapsing cancer cells' structure through inhibiting tubulin formation and reducing inflammation. canceractive.com417integrativemedicine.comhopkinsmedicine.org this compound has been shown to impede the proliferation and migration of pancreatic cancer cells. cancerchoices.orgnih.govmdpi.com Its effect on cancer cell growth and migration may be mediated through the inhibition of sphingosine kinase 1 (SK1) activity, reducing sphingosine 1-phosphate (S1P) levels. nih.govmdpi.com this compound inhibited S1P-induced cancer cell growth by regulating the JAK2/STAT3/Bcl-2 pathway and regulated cancer cell migration through the S1P/FAK/vimentin pathway. nih.govmdpi.com
This compound may have utility as a therapy after initial treatment to prevent tumor recurrence and potentially increase the durability of response to standard chemotherapy in advanced disease. hopkinsmedicine.org
Adrenocortical Cancer
Pre-clinical evidence has shown this compound's anti-cancer activity in adrenocortical cancer both in vitro and in vivo. researchgate.netparahostdis.orgecancer.orgaacrjournals.orgresearchgate.net Studies treating adrenocortical cancer cell lines (H295R and SW-13) with this compound in vitro showed dose-dependent growth arrest. ecancer.org
| Cell Line | This compound IC50 (µM) | Source |
| H295R | 0.23 | ecancer.org |
| SW-13 | 0.27 | ecancer.org |
Tumor spheroid inhibition tests at a concentration of 1 µM this compound completely disaggregated spheroids and killed cancer cells over approximately 20 days. ecancer.org
In vivo treatment of athymic nude mouse models of adrenocortical cancer with this compound significantly inhibited tumor growth and inhibited the formation of metastases. ecancer.orgresearchgate.net this compound significantly inhibited cancer cell growth both in vitro and in vivo, with effects attributed to the induction of apoptosis. researchgate.net It also inhibited invasion and migration of cancer cells in vitro and metastases formation in vivo. researchgate.net
A case report described long-term disease control in a patient with metastatic adrenocortical carcinoma treated with this compound monotherapy, with metastases initially regressing and subsequently remaining stable for an extended period. researchgate.net
Osteosarcoma
Studies have indicated the growth inhibitory effects of this compound against osteosarcoma cell lines. nih.govresearchgate.netecancer.orgnih.gov In vitro studies have reported IC50 values for this compound in osteosarcoma cell lines ranging from 0.1 to 0.8 µM. nih.govecancer.orgnih.gov this compound has shown anti-neoplastic activity against canine osteosarcoma cell lines in vitro, which are considered an excellent animal model for the human disease. nih.gov Preclinical studies suggest this compound notably impedes the growth of malignant and metastatic tumors such as osteosarcoma. researchgate.net
Gastric Cancer
This compound has demonstrated anticancer effects in gastric cancer. rjptonline.orgresearchgate.net It has been reported to hinder the development, movement, and infiltration in stomach cancer cell models. rjptonline.org
In vitro studies have shown this compound to be a potent antiproliferative agent against gastric cancer cell lines, with IC50 values ranging from 0.39 to 1.25 µM. nih.gov this compound disrupted microtubule structure and significantly inhibited invasion and migration at concentrations as low as 0.1 µM in gastric cancer cell lines. nih.gov The activity of MMP-2 significantly decreased at all tested concentrations. nih.gov
This compound was found to be more potent as an antiproliferative agent against gastric cancer cell lines than several other tested clinically approved chemotherapeutic agents. nih.gov this compound significantly induced DNA double-strand breaks in gastric cancer cells at various concentrations, comparable to doxorubicin and paclitaxel, while not causing significant damage in human lymphocytes. nih.gov It also induced G2-M arrest followed by increased caspase 3/7 activity and apoptosis. nih.gov Protein expression and mRNA levels of the proto-oncogene c-Myc were reduced by this compound exposure. nih.gov
Clinical Trials and Case Reports in Oncology
Clinical investigation into this compound as a cancer treatment has included case reports and clinical trials. While no large-scale clinical trials focused solely on this compound as a cancer treatment have been completed to date, there are documented case reports and ongoing studies exploring its potential. ecancer.org
Monotherapy Efficacy
The clinical data on this compound monotherapy for cancer is limited, primarily consisting of case reports. One notable case involved a patient with metastatic adrenocortical cancer who experienced long-term tumor control with this compound after progression despite multiple conventional treatments. ecancer.orgnih.govoup.com Preclinical studies have shown this compound to induce dose-dependent apoptosis and inhibit the growth of various cancer cell lines in vitro, including glioma, melanoma, colon cancer, and breast cancer cells, often at low micromolar concentrations. ecancer.org In vivo studies in mouse models have also demonstrated that this compound monotherapy can significantly suppress tumor growth and increase survival in models of glioma and melanoma. ecancer.orgnih.gov
Combination Therapy Regimens
This compound's potential to enhance the effects of other cancer treatments has led to its investigation in combination therapy regimens. 417integrativemedicine.com Studies suggest that this compound can synergize with a range of other drugs, including existing chemotherapeutics and ionizing radiation. ecancer.orgnih.govcanceractive.com
With Chemotherapeutic Agents
Combining this compound with chemotherapeutic agents is an area of ongoing research. Preclinical studies have indicated that this compound can enhance the cytotoxic effects of various chemotherapeutic drugs in cancer cell lines. nih.gov Clinical trials are investigating this compound in combination with standard chemotherapy regimens for specific cancer types. For instance, a Phase I/II study is evaluating this compound alongside vincristine, carboplatin, and temozolomide for pediatric low-grade gliomas, and in combination with bevacizumab and irinotecan for pediatric high-grade gliomas and diffuse intrinsic pontine gliomas. centerwatch.commycancergenome.org In metastatic colorectal cancer, adding this compound to standard chemotherapy regimens has shown promise in improving response rates and extending progression-free survival in clinical studies. 417integrativemedicine.com
With Ionizing Radiation
This compound has also been investigated for its potential to act as a radiosensitizer. In vitro studies have shown that this compound can enhance the effects of radiation therapy in various cancer cell lines, including breast cancer and meningioma cells. nih.govnih.gov This radiosensitizing effect is thought to be related to this compound's ability to disrupt microtubule formation and potentially interfere with DNA repair mechanisms. nih.govnih.gov Preclinical studies in mouse models of malignant meningioma and IDH-mutant glioma have demonstrated that the combination of this compound with radiation therapy can lead to increased apoptosis, reduced tumor growth, and improved survival compared to either treatment alone. nih.govoup.com
With Targeted Therapies (e.g., Trametinib, Metformin)
The combination of this compound with targeted therapies is another area of exploration. Preclinical evidence suggests that this compound may inhibit several targets involved in cancer progression, including VEGFR2, BRAF, and ERK, which are also targets of approved therapies like Trametinib. nih.govcanceractive.com While specific clinical trial data on this compound combined with Trametinib or Metformin was not extensively detailed in the provided information, the rationale for such combinations stems from the potential for synergistic effects based on their respective mechanisms of action. Metformin, for example, is being examined for anti-cancer effects due to its inhibition of glycolysis, a metabolic pathway often utilized by cancer cells. probes-drugs.orgguidetopharmacology.org A withdrawn Phase 3 trial listed on DrugBank included this compound and Metformin, among other drugs, for cancer treatment, although the specific outcomes are not available. drugbank.com
With Other Anthelmintics (e.g., Albendazole, Levamisole)
Here is a summary of some research findings in a table format:
| Cancer Type | Therapy Regimen | Key Findings | Source |
| Metastatic Adrenocortical Cancer | This compound Monotherapy | Case report of long-term tumor control. | ecancer.orgnih.govoup.com |
| Glioma (Preclinical) | This compound Monotherapy | Induced apoptosis, inhibited tumor growth, increased survival in mouse models. | ecancer.orgnih.gov |
| Melanoma (Preclinical) | This compound Monotherapy | Inhibited human melanoma xenograft growth in mice. | ecancer.org |
| Colorectal Cancer (Metastatic) | This compound + Standard Chemotherapy | Improved response rates and extended progression-free survival in clinical studies. | 417integrativemedicine.com |
| Pediatric Low-Grade Glioma | This compound + Vincristine, Carboplatin, Temozolomide | Investigated in Phase I/II clinical trial for safety and efficacy. | centerwatch.commycancergenome.org |
| Pediatric High-Grade Glioma | This compound + Bevacizumab, Irinotecan | Investigated in Phase I/II clinical trial for safety and efficacy. | centerwatch.commycancergenome.org |
| Malignant Meningioma (Preclinical) | This compound + Radiation Therapy | Increased apoptosis, reduced tumor growth, improved survival in rodent model. | nih.gov |
| IDH-Mutant Glioma (Preclinical) | This compound + Radiation Therapy | Increased apoptosis, reduced cell growth, disrupted cell cycle, provided survival benefit in mouse model. | oup.com |
Note: The table is intended to be interactive in a digital format, allowing for sorting and filtering.
Patient Outcomes and Survival Benefits
Research into the repurposing of this compound has explored its potential impact on patient outcomes and survival, particularly in oncology. Preclinical studies have indicated that this compound can inhibit tumor growth and enhance the effects of other cancer treatments. 417integrativemedicine.com
In preclinical models of glioblastoma multiforme (GBM), this compound demonstrated the ability to extend mean survival. In syngeneic and xenograft orthotopic mouse glioma models, this compound significantly extended mean survival by up to 63%. nih.govoup.com In a DAOY intracranial mouse xenograft model, treatment with this compound significantly increased survival from 75 days in the control group to 94 days in the group administered 25 mg/kg and 113 days in the 50 mg/kg group. nih.gov Bioluminescence imaging in this model showed a marked reduction in tumor cell proliferation. nih.gov Another study using murine GL261 glioma allografts and human medulloblastoma D425 xenografts showed that certain this compound polymorphs significantly improved mean survival. nih.gov
For metastatic colorectal cancer, preliminary evidence suggests better tumor responses, including slower disease progression, when this compound is used in combination with chemotherapy. cancerchoices.org Patients receiving this compound alongside standard chemotherapy regimens have shown improved response rates and extended progression-free survival. 417integrativemedicine.com For instance, in one instance, patients receiving this compound in combination with chemotherapy saw their median progression-free survival increase from 3 months to 9.25 months. 417integrativemedicine.com this compound may also show similar effects in metastatic adrenal cancer, although no benefit and possibly harm have been observed in individuals with unresponsive gastrointestinal cancer. cancerchoices.org
However, adding this compound to treatment with either lomustine or temozolomide for patients with recurrent glioblastoma did not have a meaningful effect on outcomes. cancerchoices.org
The following table summarizes some preclinical findings on this compound's impact on survival:
| Model | Treatment | Outcome Measured | Result | Source |
| Syngeneic and Xenograft Orthotopic Mouse Glioma | This compound | Mean Survival | Extended by up to 63% | nih.govoup.com |
| DAOY Intracranial Mouse Xenograft | This compound (25 mg/kg, 50 mg/kg) | Survival | Increased from 75 days (control) to 94 days (25mg/kg) and 113 days (50mg/kg) | nih.gov |
| Murine GL261 and Human D425 Xenografts | This compound Polymorphs (B and C) +/- Elacridar | Mean Survival | Significantly improved | nih.govmdpi.com |
This compound Repurposing in Viral Infections (e.g., COVID-19)
This compound has also garnered interest for its potential repurposing in the treatment of viral infections, including COVID-19, due to its observed antiviral and immunomodulatory properties. mdpi.com
Antiviral Effects (e.g., SARS-CoV-2)
In silico screens have identified this compound as a potential repurposed drug with antiviral activity against SARS-CoV-2. nih.govc19early.org In vitro antiviral screening has demonstrated this compound's inhibitory profile on SARS-CoV-2 viral replication at low micromolar concentrations. nih.gov this compound has shown activity against the virus in vitro. dovepress.com Studies using infected nonhuman primate cells treated with this compound showed that the compound effectively stemmed viral replication in doses similar to or significantly lower than those used clinically for other diseases, although its effects were less potent in human cell lines. news-medical.net
This compound's antiviral activity against SARS-CoV-2 is considered multifaceted. mdpi.com It is thought to arise from both direct antiviral effects and modulation of host cellular machinery. mdpi.com this compound inhibits tubulin polymerization by binding to β-tubulin, disrupting microtubule dynamics essential for intracellular transport, which viruses, including coronaviruses, rely on for replication and propagation. mdpi.com this compound also has the ability to induce apoptosis via the dysregulation of Poly (ADP-ribose) Polymerase (PARP) and the activation of the cytoplasmic DNA sensor (cGAS). nih.gov It may also inhibit COVID-19 viral trafficking by impairing cellular microtubule integrity. nih.gov this compound has been shown to inhibit heat shock protein 90 (Hsp90), a molecular chaperone involved in the folding and stabilization of several host and viral proteins. mdpi.com
Immunomodulatory Effects in Viral Contexts
This compound may exert immunomodulatory effects by downregulating pro-inflammatory cytokines such as IL-6 and TNF-α, which are implicated in the cytokine storm associated with severe COVID-19. mdpi.com This anti-inflammatory action may help reduce pulmonary inflammation and tissue damage in infected individuals. mdpi.com this compound may modulate the host immune response to prevent viral multiplication and lower inflammation. mdpi.com In a clinical trial involving COVID-19 outpatients, this compound therapy increased innate immunity and returned inflammation to normal levels more quickly than in the placebo group. c19early.orgnih.govresearchgate.net This was evidenced by lower CRP levels in the treatment group compared to placebo. mdpi.comc19early.orgnih.gov
Impact on Viral Replication and Clearance
In a randomized, placebo-controlled clinical trial involving outpatients with mild to moderate COVID-19, this compound treatment was associated with significant improvements in virological and inflammatory markers by day three of therapy. mdpi.com Cycle threshold (CT) values, a proxy for viral load, were significantly higher in the this compound group compared with placebo, indicating a lower viral load. mdpi.comc19early.orgnih.gov Within the this compound group, CT values also increased significantly from baseline, further supporting a reduction in viral burden. mdpi.comc19early.orgnih.gov Changes in primary clinical endpoints, such as CT and white blood cell differential, suggest faster viral clearance in the this compound group compared to the placebo group. nih.govresearchgate.net The reduction and normalization of monocytes may also indicate a more rapid eradication of the infection and its associated inflammation. nih.gov
An observational retrospective study of PCR-confirmed COVID-19 patients who received this compound showed that for the inpatient cohort, the hospital length of stay was significantly shorter in the treatment group compared to the control group. nih.govc19early.org Adjusting for covariates, the hospital length of stay was 3.5 days shorter in the treatment group. nih.govc19early.org There were also fewer patients who required either invasive or noninvasive ventilation in the treatment group, and the mortality rate was lower, although these differences did not reach statistical significance. nih.govc19early.org
Exploration in Other Conditions (e.g., Ulcerative Colitis)
Beyond cancer and viral infections, this compound is also being explored for its potential in treating other conditions, such as ulcerative colitis. clinicaltrials.eu this compound has known anti-inflammatory properties. figshare.comresearchgate.net
A study in a murine model of DSS-induced colitis explored the protective effects of this compound. figshare.comresearchgate.net this compound significantly improved the colitis disease activity index, as assessed by changes in body weight, stool consistency, rectal bleeding, and prolapse. figshare.comresearchgate.net It also ameliorated the colon histopathological score by attenuating crypt loss, mucosal damage, and inflammation score in colitis tissues. figshare.comresearchgate.net DSS-induced colon shortening, colon weight loss, and increase in spleen weight were all abrogated in the presence of this compound. figshare.comresearchgate.net this compound decreased inflammation, possibly by reducing oxidative stress markers, suppressing inflammatory cell infiltration, and down-regulation of inflammatory genes in colon tissues. figshare.comresearchgate.net Furthermore, it reduced fibrosis by decreasing collagen deposition and down-regulating pro-fibrotic genes. figshare.comresearchgate.net This study suggested that this compound could be repurposed as a novel therapy for ulcerative colitis. figshare.comresearchgate.net
Conversely, a population-based cohort study using data from Denmark between 1995 and 2018 investigated the association between early-life this compound exposure and the risk of adult-onset inflammatory bowel disease (IBD). mimsdev.comdr-efi.com The study found that exposure to this compound prior to the age of 5 was associated with a significantly elevated risk of adult-onset IBD, specifically ulcerative colitis. mimsdev.comdr-efi.com The adjusted hazard ratio for adult-onset IBD when limiting exposure to early life (<5 years) was 1.17, with a 95% confidence interval of 1.04–1.31. mimsdev.com This increased risk was driven by ulcerative colitis (aHR, 1.32; 95% CI, 1.12–1.55), but not Crohn's disease. mimsdev.com Exposure at less than 18 years of age was not significantly associated with either pediatric- or adult-onset IBD. mimsdev.com These findings suggest the potential importance of early-life exposures in shaping the risk of IBD later in life. mimsdev.com
The following table summarizes findings regarding this compound and ulcerative colitis:
| Study Type | Model/Population | Key Findings | Source |
| Preclinical Study | Murine model of DSS-induced colitis | Improved colitis disease activity index and histopathological score; decreased inflammation and fibrosis. | figshare.comresearchgate.net |
| Population-Based Cohort Study | Individuals born in Denmark (1995-2018) | Early-life exposure (<5 years) associated with significantly elevated risk of adult-onset ulcerative colitis (aHR, 1.32; 95% CI, 1.12–1.55). No association with exposure after age 5 or with pediatric-onset IBD or adult-onset Crohn's disease. | mimsdev.comdr-efi.com |
Future Directions and Research Gaps
Elucidating Novel Molecular Targets and Pathways
While mebendazole is known to primarily target tubulin polymerization in parasites and cancer cells, there is a need to fully elucidate its broader molecular targets and the complex pathways it influences. Research using in silico target prediction algorithms has suggested multiple putative molecular targets for this compound, including proteins significantly upregulated in certain cancers like glioblastoma. biorxiv.orgcapes.gov.brnih.gov Validation experiments have shown that this compound can inhibit the activity of kinases such as ABL1, MAPK1/ERK2, and MAPK14/p38α in vitro, with particularly high potency against MAPK14. biorxiv.orgcapes.gov.brnih.govresearchgate.net Molecular modeling supports the idea that this compound can bind to the catalytic site of MAPK14. biorxiv.orgnih.govresearchgate.net Further investigation into how this compound regulates pathways like the S1P/JAK2/STAT3 and S1P/FAK/Vimentin signaling pathways in specific cancer types, such as pancreatic cancer, is essential to understand its complete mechanism of action and identify potential new therapeutic avenues or biomarkers for response. mdpi.com Studies have also indicated that this compound can influence the Girdin-mediated Akt/IKKα/β/NF-κB signaling axis in ovarian cancer, highlighting novel mechanisms beyond tubulin binding. mdpi.com Understanding these diverse molecular interactions is crucial for predicting efficacy, identifying potential drug combinations, and overcoming resistance.
Overcoming Drug Resistance Mechanisms in Cancer and Parasitic Infections
Drug resistance poses a significant challenge in both cancer therapy and the control of parasitic infections, and this is also a concern for this compound. In the context of cancer, this compound has shown activity against certain chemoresistant cell lines, including cisplatin-resistant ovarian cancer and chemoresistant melanoma. aging-us.comaacrjournals.orgeurekalert.orgnih.gov Mechanistically, this compound has been shown to inhibit multiple cancer-related signaling pathways in cisplatin-resistant ovarian cancer cells, such as ELK/SRF, NFKB, MYC/MAX, and E2F/DP1. aging-us.com However, the specific mechanisms by which cancer cells develop resistance to this compound itself require further investigation. Similarly, in parasitic infections, the occurrence of drug resistance to this compound has been reported in hookworm infections, trichuriasis, ascariasis, and lymphatic filariasis, becoming a significant emerging issue. nih.gov The low efficacy of single-dose this compound treatment against hookworms in some areas may be due to a general low susceptibility or local resistance. asm.org Research is needed to understand the genetic and molecular basis of this compound resistance in various helminth species and to develop strategies, including novel drug combinations or formulations, to circumvent these resistance mechanisms. asm.org
Development of Novel Formulations and Delivery Systems (e.g., Nanoparticles)
This compound's poor aqueous solubility and low oral bioavailability limit its systemic exposure, which is particularly relevant for treating disseminated cancers or systemic parasitic infections. tandfonline.compreprints.orgrjptonline.org The development of novel formulations and delivery systems is a critical area for future research. Nanocrystal formulations, for instance, are being investigated to enhance the dissolution properties of this compound. tandfonline.com Studies have shown that nanocrystal formulations can exhibit significant dissolution enhancement compared to neat this compound. tandfonline.com Polymeric nanocapsules containing this compound have also been explored, demonstrating potential for enhanced efficacy in treating hydatid cysts in mice, likely due to improved drug absorption. mdpi.com Other novel oral formulations, such as β-cyclodextrin inclusion complexes and chitosan-based microcrystals, have shown improved in vitro solubility and enhanced in vivo activity against hookworms in animal models compared to standard this compound. researchgate.netnih.gov Further research is needed to optimize these formulations, evaluate their pharmacokinetics and safety in humans, and assess their ability to deliver effective concentrations of this compound to target tissues, including the brain for central nervous system malignancies. nih.govnih.govspringermedizin.de
Strategic Combination Therapies for Enhanced Efficacy
Combining this compound with other therapeutic agents holds significant promise for enhancing efficacy and potentially overcoming resistance in both cancer and parasitic diseases. Preclinical studies have shown that this compound can synergize with various agents, including standard chemotherapeutics and targeted therapies in cancer models. capes.gov.brnih.govresearchgate.netmdpi.comaging-us.commdpi.com417integrativemedicine.comecancer.orgbrad.ac.ukoup.comresearchgate.netaacrjournals.orgoncotarget.comnih.gov For example, combining this compound with elacridar, a P-glycoprotein inhibitor, has shown improved efficacy in mouse brain tumor models, suggesting a potential role in enhancing brain penetration or reducing efflux. nih.govaacrjournals.orgnih.gov this compound has also demonstrated synergy with cisplatin in overcoming resistance in ovarian cancer cells and with trametinib in suppressing melanoma growth in xenograft models. aging-us.comoncotarget.com In parasitic infections, combined treatment with this compound and levamisole has shown higher efficacy against hookworm infections compared to either drug alone, and this combination may also help delay the development of benzimidazole resistance. paediatricaindonesiana.org Future research should focus on rationally designed combination strategies based on the elucidated molecular targets and resistance mechanisms, evaluating efficacy and safety in relevant preclinical models and translating promising combinations into clinical trials.
Large-Scale Clinical Trials for Repurposed Indications
While preclinical data and early-phase clinical trials investigating this compound for repurposed indications, particularly in oncology, are encouraging, large-scale, well-controlled clinical trials are crucial to definitively establish its efficacy and safety in these new contexts. This compound is currently being investigated in clinical trials for various potential uses beyond parasitic infections, including brain tumors, high-grade gliomas, liver cancer, and ulcerative colitis. clinicaltrials.eu Clinical studies have explored its use in pediatric brain tumors and as part of combination therapies for soil-transmitted helminth infections. clinicaltrials.eu There is evidence suggesting that candidate cancer types for human trials include melanoma, non-small cell lung cancer, adrenocortical cancer, and colon cancer. ecancer.orgnih.gov Further large-scale trials are needed across different cancer types and stages to determine optimal dosing regimens, evaluate efficacy as a monotherapy and in combination with standard treatments, and identify specific patient populations who are most likely to benefit. oup.commdpi.comresearchgate.net
Q & A
Basic Research Question: What experimental design considerations are critical for evaluating mebendazole’s pharmacokinetics in heterogeneous populations?
Methodological Answer:
To assess pharmacokinetics (PK) in diverse populations, researchers should:
- Define subpopulations (e.g., neonates, immunocompromised individuals) based on metabolic or physiological differences .
- Use population PK modeling to account for variability in drug absorption, distribution, and clearance. For example, sparse sampling in pediatric cohorts can reduce ethical and logistical challenges .
- Validate assays (e.g., HPLC, differential pulse polarography) to ensure sensitivity in detecting low plasma concentrations .
- Data sharing protocols must comply with ethical standards, including anonymization and controlled access to sensitive datasets .
Advanced Research Question: How can conflicting efficacy data for this compound in repurposed oncology studies be reconciled?
Methodological Answer:
Conflicting results often arise from:
- Variability in experimental models : Compare outcomes across cell lines (e.g., LNCaP vs. DU145 prostate cancer cells) and in vivo models (e.g., xenografts vs. genetically engineered mice) .
- Dose-response discordance : Use dose-ranging studies to identify therapeutic thresholds. For example, this compound’s anti-cancer effects in PDE4D7-knockdown LNCaPs occur at lower doses than in wild-type cells .
- Mechanistic heterogeneity : Conduct transcriptomic or proteomic analyses to map pathways (e.g., cAMP dynamics, microtubule disruption) influenced by tumor microenvironment factors .
- Meta-analysis frameworks : Apply PRISMA guidelines to aggregate preclinical data and identify bias sources (e.g., publication bias, model selection) .
Basic Research Question: What validated analytical methods are recommended for quantifying this compound in pharmaceutical formulations?
Methodological Answer:
- Electrochemical techniques : Differential pulse polarography (DPP) offers sensitivity at µg/mL levels, validated in pH 7.4 buffers to mimic physiological conditions .
- Chromatography : HPLC with UV detection (λ = 254 nm) provides reproducibility, but requires column optimization to separate this compound from excipients .
- Quality control : Cross-validate results with mass spectrometry (LC-MS) to confirm specificity, especially in complex matrices like serum .
Advanced Research Question: What strategies address this compound’s solubility limitations in preclinical testing?
Methodological Answer:
- Co-solvent systems : Test biocompatible solvents (e.g., PEG-400) to enhance aqueous solubility while monitoring cytotoxicity in vitro .
- Nanoformulation : Develop liposomal or polymeric nanoparticles to improve bioavailability. Characterize particle size (DLS) and encapsulation efficiency (UV-Vis) .
- In silico modeling : Use tools like COSMO-RS to predict solubility in simulated biological fluids and guide formulation design .
Basic Research Question: How should researchers design studies to evaluate this compound resistance in helminthic parasites?
Methodological Answer:
- Longitudinal sampling : Collect parasite isolates pre- and post-treatment to track β-tubulin mutations linked to resistance .
- Phenotypic assays : Measure IC50 shifts in larval motility or egg hatching inhibition assays across multiple generations .
- Genomic sequencing : Identify SNPs in β-tubulin isotype-1 genes and correlate with clinical failure rates .
Advanced Research Question: What methodologies optimize this compound combination therapies for synergistic anti-helminthic effects?
Methodological Answer:
- Checkerboard assays : Determine fractional inhibitory concentration indices (FICI) for this compound paired with albendazole or ivermectin .
- Mechanistic synergy : Use RNAi or CRISPR to validate target pathways (e.g., dual β-tubulin and glutamate-gated chloride channel disruption) .
- In vivo validation : Employ factorial design experiments in rodent models to assess efficacy-toxicity trade-offs .
Basic Research Question: How to ensure reproducibility in this compound’s in vitro cytotoxicity assays?
Methodological Answer:
- Standardize cell lines : Use authenticated stocks (e.g., ATCC-certified DU145) and control for passage number .
- Culture conditions : Maintain consistent O2 levels (5% CO2) and serum concentrations (10% FBS) to minimize batch effects .
- Endpoint validation : Combine MTT assays with live-cell imaging to confirm apoptosis vs. necrosis .
Advanced Research Question: How can computational models predict this compound’s off-target effects in repurposing studies?
Methodological Answer:
- Docking simulations : Use AutoDock Vina to screen this compound against human kinases or GPCRs implicated in side effects .
- Network pharmacology : Construct protein-protein interaction networks to identify secondary targets (e.g., PDE4D7 in prostate cancer) .
- Toxicogenomics : Apply LINCS database queries to predict gene expression changes in non-target tissues .
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Featured Recommendations
| Most viewed | ||
|---|---|---|
| Most popular with customers |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


