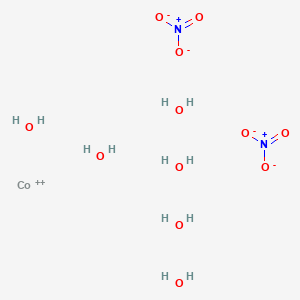
Cobaltous nitrate hexahydrate
Cat. No. B158006
Key on ui cas rn:
10026-22-9
M. Wt: 139.962 g/mol
InChI Key: YKPDYTLBLKQHDA-UHFFFAOYSA-N
Attention: For research use only. Not for human or veterinary use.
Patent
US05981445
Procedure details


21.6 g (0.102) of strontium nitrate was dissolved in about 60 ml of distilled water, and this solution was poured over 32.26 g (0.099 mole) lanthanum oxide in a 150 cm3 beaker and left to react. Meanwhile 27.92 g nickel nitrate hexahydrate (0.096 mole) and 55.1 g (0.189 mole) cobalt nitrate hexahydrate and 6.06 g (0.015 mole) iron nitrate nanohydrate were dissolved in about 140 cm3 distilled water. The slurry of lanthanum hydroxide in strontium nitrate solution, resulting from reaction of lanthanum oxide with water was then quantitatively blended under vigorous stirring into the solution of nickel and cobalt nitrates. The resulting mixture was further vigorously stirred for about one hour until pourable homogeneous dark green suspension of insoluble metal hydroxides was obtained. This mixture was then further processed by spray-freezing, freeze-drying, dry-milling for about one hour of the freeze-dried agglomerates, and calcination as described in the example 1. The specific surface area of the resulting powder was 9.5 m2 /g. This powder has been further processed by wet (water suspension) milling, addition of colloidal silica to the suspension, and depositing the perovskite powder slurry on pretreated alumina pellets. The loading of perovskite powder was 7.5%, and the catalyst showed a good catalytic activity for methane combustion (FIG. 3) as well as for toluene oxidation. In preparing 0.3 mole La0.66 Sr0.34 Ni0.32 Co0.63 Fe0.05 O3, 0.819 mole (corresponding to 16.8 liters) NOx were produced, e.g. 2.73 mole NOx /1 mole perovskite powder.



Name
nickel nitrate hexahydrate
Quantity
27.92 g
Type
catalyst
Reaction Step Three



Identifiers


|
REACTION_CXSMILES
|
[N+]([O-])([O-])=[O:2].[Sr+2].[N+]([O-])([O-])=[O:7].[O-2:10].[La+3:11].[O-2].[O-2].[La+3]>O.O.O.O.O.O.O.[N+]([O-])([O-])=O.[Ni+2].[N+]([O-])([O-])=O.O.O.O.O.O.O.[N+]([O-])([O-])=O.[Co+2].[N+]([O-])([O-])=O.[N+]([O-])([O-])=O.[Fe+2].[N+]([O-])([O-])=O>[OH-:2].[La+3:11].[OH-:7].[OH-:10].[O-2:2].[La+3:11].[O-2:2].[O-2:2].[La+3:11] |f:0.1.2,3.4.5.6.7,9.10.11.12.13.14.15.16.17,18.19.20.21.22.23.24.25.26,27.28.29,30.31.32.33,34.35.36.37.38|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
21.6 g
|
|
Type
|
reactant
|
|
Smiles
|
[N+](=O)([O-])[O-].[Sr+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
60 mL
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Two
|
Name
|
|
|
Quantity
|
32.26 g
|
|
Type
|
reactant
|
|
Smiles
|
[O-2].[La+3].[O-2].[O-2].[La+3]
|
Step Three
|
Name
|
nickel nitrate hexahydrate
|
|
Quantity
|
27.92 g
|
|
Type
|
catalyst
|
|
Smiles
|
O.O.O.O.O.O.[N+](=O)([O-])[O-].[Ni+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
55.1 g
|
|
Type
|
catalyst
|
|
Smiles
|
O.O.O.O.O.O.[N+](=O)([O-])[O-].[Co+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
6.06 g
|
|
Type
|
catalyst
|
|
Smiles
|
[N+](=O)([O-])[O-].[Fe+2].[N+](=O)([O-])[O-]
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


WAIT
|
Type
|
WAIT
|
|
Details
|
left
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to react
|
DISTILLATION
|
Type
|
DISTILLATION
|
|
Details
|
distilled water
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
[OH-].[La+3].[OH-].[OH-]
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
[O-2].[La+3].[O-2].[O-2].[La+3]
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US05981445
Procedure details


21.6 g (0.102) of strontium nitrate was dissolved in about 60 ml of distilled water, and this solution was poured over 32.26 g (0.099 mole) lanthanum oxide in a 150 cm3 beaker and left to react. Meanwhile 27.92 g nickel nitrate hexahydrate (0.096 mole) and 55.1 g (0.189 mole) cobalt nitrate hexahydrate and 6.06 g (0.015 mole) iron nitrate nanohydrate were dissolved in about 140 cm3 distilled water. The slurry of lanthanum hydroxide in strontium nitrate solution, resulting from reaction of lanthanum oxide with water was then quantitatively blended under vigorous stirring into the solution of nickel and cobalt nitrates. The resulting mixture was further vigorously stirred for about one hour until pourable homogeneous dark green suspension of insoluble metal hydroxides was obtained. This mixture was then further processed by spray-freezing, freeze-drying, dry-milling for about one hour of the freeze-dried agglomerates, and calcination as described in the example 1. The specific surface area of the resulting powder was 9.5 m2 /g. This powder has been further processed by wet (water suspension) milling, addition of colloidal silica to the suspension, and depositing the perovskite powder slurry on pretreated alumina pellets. The loading of perovskite powder was 7.5%, and the catalyst showed a good catalytic activity for methane combustion (FIG. 3) as well as for toluene oxidation. In preparing 0.3 mole La0.66 Sr0.34 Ni0.32 Co0.63 Fe0.05 O3, 0.819 mole (corresponding to 16.8 liters) NOx were produced, e.g. 2.73 mole NOx /1 mole perovskite powder.



Name
nickel nitrate hexahydrate
Quantity
27.92 g
Type
catalyst
Reaction Step Three



Identifiers


|
REACTION_CXSMILES
|
[N+]([O-])([O-])=[O:2].[Sr+2].[N+]([O-])([O-])=[O:7].[O-2:10].[La+3:11].[O-2].[O-2].[La+3]>O.O.O.O.O.O.O.[N+]([O-])([O-])=O.[Ni+2].[N+]([O-])([O-])=O.O.O.O.O.O.O.[N+]([O-])([O-])=O.[Co+2].[N+]([O-])([O-])=O.[N+]([O-])([O-])=O.[Fe+2].[N+]([O-])([O-])=O>[OH-:2].[La+3:11].[OH-:7].[OH-:10].[O-2:2].[La+3:11].[O-2:2].[O-2:2].[La+3:11] |f:0.1.2,3.4.5.6.7,9.10.11.12.13.14.15.16.17,18.19.20.21.22.23.24.25.26,27.28.29,30.31.32.33,34.35.36.37.38|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
21.6 g
|
|
Type
|
reactant
|
|
Smiles
|
[N+](=O)([O-])[O-].[Sr+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
60 mL
|
|
Type
|
solvent
|
|
Smiles
|
O
|
Step Two
|
Name
|
|
|
Quantity
|
32.26 g
|
|
Type
|
reactant
|
|
Smiles
|
[O-2].[La+3].[O-2].[O-2].[La+3]
|
Step Three
|
Name
|
nickel nitrate hexahydrate
|
|
Quantity
|
27.92 g
|
|
Type
|
catalyst
|
|
Smiles
|
O.O.O.O.O.O.[N+](=O)([O-])[O-].[Ni+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
55.1 g
|
|
Type
|
catalyst
|
|
Smiles
|
O.O.O.O.O.O.[N+](=O)([O-])[O-].[Co+2].[N+](=O)([O-])[O-]
|
|
Name
|
|
|
Quantity
|
6.06 g
|
|
Type
|
catalyst
|
|
Smiles
|
[N+](=O)([O-])[O-].[Fe+2].[N+](=O)([O-])[O-]
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


WAIT
|
Type
|
WAIT
|
|
Details
|
left
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
to react
|
DISTILLATION
|
Type
|
DISTILLATION
|
|
Details
|
distilled water
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
[OH-].[La+3].[OH-].[OH-]
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
[O-2].[La+3].[O-2].[O-2].[La+3]
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
