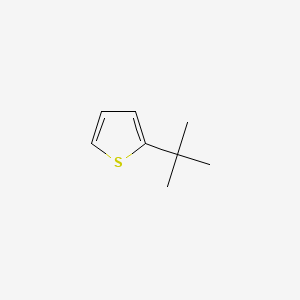
2-tert-Butylthiophene
Cat. No. B1664577
Key on ui cas rn:
1689-78-7
M. Wt: 140.25 g/mol
InChI Key: SWCDOJGIOCVXFM-UHFFFAOYSA-N
Attention: For research use only. Not for human or veterinary use.
Patent
US05863419
Procedure details


The alkylation process results in the substitution of an alkyl group for a hydrogen atom in the sulfur-containing starting material and causes a corresponding increase in molecular weight over that of the starting material. The higher molecular weight of such an alkylation product is reflected by a higher boiling point relative to that of the starting material. For example, the conversion of thiophene to 2-t-butylthiophene by alkylation with 2-methylpropene results in the conversion of thiophene, which has a boiling point of 84° C., to a product which has a boiling point of 164° C. and can be easily removed from lower boiling material in the feedstock by fractional distillation. Conversion of thiophene to di-t-butylthiophene by dialkylation with 2-methylpropene results in a product which has an even higher boiling point of about 224° C. Alkylation with alkyl groups that add a large rather than a small number of carbon atoms is preferred since the products will have higher molecular weights and, accordingly, will usually have higher boiling points than products which are obtained through alkylation with the smaller alkyl groups.





Name

Identifiers


|
REACTION_CXSMILES
|
[S].[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:7]([C:11]1[S:12][CH:13]=[CH:14][CH:15]=1)([CH3:10])([CH3:9])[CH3:8].[CH3:16][C:17]([CH3:19])=[CH2:18]>>[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:17]([C:15]1[CH:14]=[CH:13][S:12][C:11]=1[C:7]([CH3:10])([CH3:9])[CH3:8])([CH3:19])([CH3:18])[CH3:16].[CH3:9][C:7]([CH3:10])=[CH2:8] |^3:0|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[S]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)(C)C=1SC=CC1
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC(=C)C
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
a corresponding increase in molecular weight over that of the starting material
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
can be easily removed from lower boiling material in the feedstock by fractional distillation
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
S1C=CC=C1
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C)(C)(C)C1=C(SC=C1)C(C)(C)C
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US05863419
Procedure details


The alkylation process results in the substitution of an alkyl group for a hydrogen atom in the sulfur-containing starting material and causes a corresponding increase in molecular weight over that of the starting material. The higher molecular weight of such an alkylation product is reflected by a higher boiling point relative to that of the starting material. For example, the conversion of thiophene to 2-t-butylthiophene by alkylation with 2-methylpropene results in the conversion of thiophene, which has a boiling point of 84° C., to a product which has a boiling point of 164° C. and can be easily removed from lower boiling material in the feedstock by fractional distillation. Conversion of thiophene to di-t-butylthiophene by dialkylation with 2-methylpropene results in a product which has an even higher boiling point of about 224° C. Alkylation with alkyl groups that add a large rather than a small number of carbon atoms is preferred since the products will have higher molecular weights and, accordingly, will usually have higher boiling points than products which are obtained through alkylation with the smaller alkyl groups.





Name

Identifiers


|
REACTION_CXSMILES
|
[S].[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:7]([C:11]1[S:12][CH:13]=[CH:14][CH:15]=1)([CH3:10])([CH3:9])[CH3:8].[CH3:16][C:17]([CH3:19])=[CH2:18]>>[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:17]([C:15]1[CH:14]=[CH:13][S:12][C:11]=1[C:7]([CH3:10])([CH3:9])[CH3:8])([CH3:19])([CH3:18])[CH3:16].[CH3:9][C:7]([CH3:10])=[CH2:8] |^3:0|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[S]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)(C)C=1SC=CC1
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC(=C)C
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
a corresponding increase in molecular weight over that of the starting material
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
can be easily removed from lower boiling material in the feedstock by fractional distillation
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
S1C=CC=C1
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C)(C)(C)C1=C(SC=C1)C(C)(C)C
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US05863419
Procedure details


The alkylation process results in the substitution of an alkyl group for a hydrogen atom in the sulfur-containing starting material and causes a corresponding increase in molecular weight over that of the starting material. The higher molecular weight of such an alkylation product is reflected by a higher boiling point relative to that of the starting material. For example, the conversion of thiophene to 2-t-butylthiophene by alkylation with 2-methylpropene results in the conversion of thiophene, which has a boiling point of 84° C., to a product which has a boiling point of 164° C. and can be easily removed from lower boiling material in the feedstock by fractional distillation. Conversion of thiophene to di-t-butylthiophene by dialkylation with 2-methylpropene results in a product which has an even higher boiling point of about 224° C. Alkylation with alkyl groups that add a large rather than a small number of carbon atoms is preferred since the products will have higher molecular weights and, accordingly, will usually have higher boiling points than products which are obtained through alkylation with the smaller alkyl groups.





Name

Identifiers


|
REACTION_CXSMILES
|
[S].[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:7]([C:11]1[S:12][CH:13]=[CH:14][CH:15]=1)([CH3:10])([CH3:9])[CH3:8].[CH3:16][C:17]([CH3:19])=[CH2:18]>>[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:17]([C:15]1[CH:14]=[CH:13][S:12][C:11]=1[C:7]([CH3:10])([CH3:9])[CH3:8])([CH3:19])([CH3:18])[CH3:16].[CH3:9][C:7]([CH3:10])=[CH2:8] |^3:0|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[S]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)(C)C=1SC=CC1
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC(=C)C
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
a corresponding increase in molecular weight over that of the starting material
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
can be easily removed from lower boiling material in the feedstock by fractional distillation
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
S1C=CC=C1
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C)(C)(C)C1=C(SC=C1)C(C)(C)C
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
Patent
US05863419
Procedure details


The alkylation process results in the substitution of an alkyl group for a hydrogen atom in the sulfur-containing starting material and causes a corresponding increase in molecular weight over that of the starting material. The higher molecular weight of such an alkylation product is reflected by a higher boiling point relative to that of the starting material. For example, the conversion of thiophene to 2-t-butylthiophene by alkylation with 2-methylpropene results in the conversion of thiophene, which has a boiling point of 84° C., to a product which has a boiling point of 164° C. and can be easily removed from lower boiling material in the feedstock by fractional distillation. Conversion of thiophene to di-t-butylthiophene by dialkylation with 2-methylpropene results in a product which has an even higher boiling point of about 224° C. Alkylation with alkyl groups that add a large rather than a small number of carbon atoms is preferred since the products will have higher molecular weights and, accordingly, will usually have higher boiling points than products which are obtained through alkylation with the smaller alkyl groups.





Name

Identifiers


|
REACTION_CXSMILES
|
[S].[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:7]([C:11]1[S:12][CH:13]=[CH:14][CH:15]=1)([CH3:10])([CH3:9])[CH3:8].[CH3:16][C:17]([CH3:19])=[CH2:18]>>[S:2]1[CH:6]=[CH:5][CH:4]=[CH:3]1.[C:17]([C:15]1[CH:14]=[CH:13][S:12][C:11]=1[C:7]([CH3:10])([CH3:9])[CH3:8])([CH3:19])([CH3:18])[CH3:16].[CH3:9][C:7]([CH3:10])=[CH2:8] |^3:0|
|
Inputs


Step One
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
[S]
|
Step Two
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Step Three
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
C(C)(C)(C)C=1SC=CC1
|
Step Four
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
CC(=C)C
|
Step Five
|
Name
|
|
|
Quantity
|
0 (± 1) mol
|
|
Type
|
reactant
|
|
Smiles
|
S1C=CC=C1
|
Conditions


Other
|
Conditions are dynamic
|
1
|
|
Details
|
See reaction.notes.procedure_details.
|
Workups


ADDITION
|
Type
|
ADDITION
|
|
Details
|
containing
|
TEMPERATURE
|
Type
|
TEMPERATURE
|
|
Details
|
a corresponding increase in molecular weight over that of the starting material
|
CUSTOM
|
Type
|
CUSTOM
|
|
Details
|
can be easily removed from lower boiling material in the feedstock by fractional distillation
|
Outcomes


Product
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
S1C=CC=C1
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
C(C)(C)(C)C1=C(SC=C1)C(C)(C)C
|
|
Name
|
|
|
Type
|
product
|
|
Smiles
|
CC(=C)C
|
Source


|
Source
|
Open Reaction Database (ORD) |
|
Description
|
The Open Reaction Database (ORD) is an open-access schema and infrastructure for structuring and sharing organic reaction data, including a centralized data repository. The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. Our vision is that a consistent data representation and infrastructure to support data sharing will enable downstream applications that will greatly improve the state of the art with respect to computer-aided synthesis planning, reaction prediction, and other predictive chemistry tasks. |
